Polyvinyl chloride (PVC) possesses outstanding corrosion resistance, flame retardancy, and high mechanical properties, and is thus widely used in industries such as construction, electronics, packaging, and pipe manufacturing. However, as a manufacturer specializing in PVC processing, we are well aware of the key challenges that exist in the production process of PVC: Due to the double bonds, branching points, and initiator residues in its structure, when heated to 100°C, PVC begins to degrade – accompanied by a dechlorination hydrogen reaction; when the temperature reaches the processing level (170°C or higher), this degradation accelerates sharply, and a rapid high-molecular cross-linking reaction occurs, directly leading to product discoloration, performance degradation, and reduced production efficiency. To address these issues, stabilizers are indispensable in PVC formulations. By definition, stabilizers are substances added to polymers to enhance their thermal stability; in a broad sense, any material aimed at improving the thermal stability of polymers can be called a thermal stabilizer.
This article focuses on traditional thermal stabilizers – these are essential additives in the processing of polyvinyl chloride. These additives not only prevent degradation caused by high temperatures and mechanical shearing during production, but also ensure good long-term weather resistance throughout the processing and storage. Next, we will start from the classification and performance requirements of stabilizers, delve into the degradation and stabilization mechanisms of polyvinyl chloride, systematically sort out the action principles and synergistic effects of various stabilizers (such as lead salts, metal soaps, organic tin, and rare earth compounds), and share key insights into formulation design, providing scientific and practical technical references for your polyvinyl chloride production.
- 1. Classification and Performance Requirements of Thermal Stabilizers
- 2. Degradation and Stabilization of Polyvinyl Chloride
- Conclusion
1. Classification and Performance Requirements of Thermal Stabilizers
An ideal thermal stabilizer should meet the following basic usage conditions (selection principles):
(1) High thermal stability and good light stability.
(2) Good compatibility with PVC, good volatility, no volatilization, no migration, no frosting, and not easily extracted by water, oil, or other solvents.
(3) Appropriate lubricity, no segregation during extrusion, no fouling.
(4) No reaction with other additives, not contaminated by sulfur and copper and other substances.
(5) Does not reduce the secondary processing properties of the product, such as electrical properties and printability.
(6) Non-toxic, no abnormal odor, no pollution, and can produce transparent products.
(7) Convenient for processing and low cost.
In reality, there is no single stabilizer that can fully meet these conditions. Therefore, it is necessary to combine and select stabilizers according to needs. There are many types of thermal stabilizers for PVC, and classification methods vary, but chemical composition classification is generally used. It includes lead salts, metal soaps, organic tin compounds, organic antimony compounds, rare earth compounds, and organic auxiliary stabilizers.
2. Degradation and Stabilization of Polyvinyl Chloride
2.1 Degradation Phenomenon of PVC
From the high thermal stability of 1,3,5-trichlorohexane, it can be seen that PVC with a similar basic structure should also be very stable. However, this is not the case. PVC is far less stable than simple chlorides. When heated to 100°C, PVC undergoes a violent degradation accompanied by dehydrochlorination. At 100-200°C during processing, PVC undergoes large molecule cross-linking, causing the product to turn black and deteriorating its physical properties. The degradation of PVC is accompanied by double bonds produced by dehydrochlorination, activating the chlorine atoms on the carbon atoms, causing the release of HCl molecules. The presence of oxygen accelerates the degradation of PVC.
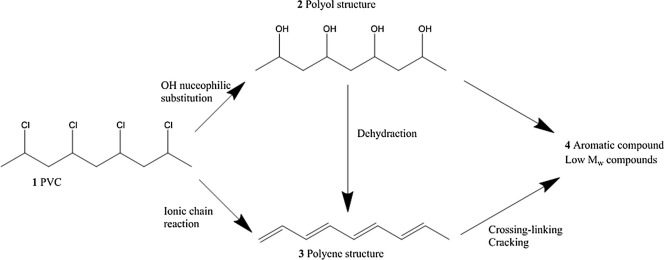
2.2 Degradation Mechanism of PVC and Factors Affecting PVC Degradation
The commonly accepted degradation theories in the PVC industry include free radical mechanism, ionic mechanism, and monomolecular mechanism. The free radical mechanism is the most popular. Generally, there are eight factors that affect PVC degradation, namely, the influence of structure, the influence of oxygen, the influence of HCl, the influence of metal chlorides, the influence of solvents, the influence of plasticizers, and the influence of critical size. Some unforeseen factors also exist.
2.3 Inhibition of Degradation
To effectively prevent the degradation of PVC, it is necessary to control the degradation process of the degradation force acting on the polymer system during a certain period. The purpose of stabilizers is to achieve comprehensive control of PVC’s color, rheological properties, mechanical properties, electrical properties, chemical resistance, thermal properties, optical properties, etc., through the control of degradation force and degradation process. In actual production, the realization of PVC stability mainly relies on two approaches. One is to strictly control the production process to ensure that the resulting product has as high stability as possible. This method is generally referred to as Preventive Stabilization Technology. The other method is to add one or more substances before or during the processing of the resin to inhibit the degradation that has already begun. This method is generally called Degradation-Terminating Stabilization Technology. The underlying principle of this method is precisely the function of heat stabilizers.
2.4 The Action Mechanism of Heat Stabilizers
2.4.1 Lead Salts
Most of these stabilizers are lead salts containing Basic Group (PbO). Due to PbO’s extremely strong ability to neutralize HCl, their main function is to neutralize the HCl generated during the degradation of PVC.
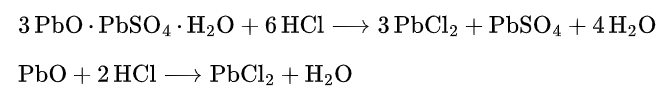
2.4.2 Metal Soaps
(1) Reaction with HCl
Their reaction with HCL is the basic reaction of heat stabilizers. The metal in Carboxylic Acid metal soaps is generally divalent, so there are two steps of reaction:
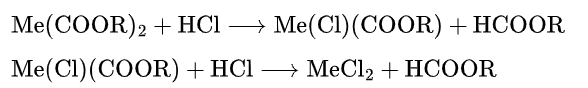
Due to the high covalent tendency of zinc and the presence of unshared electron pairs, and the higher reactivity of allylic chlorine compared to other parts of the polymer chain, the allylic group combines with the unstable zinc intermediate. For cadmium soaps, the reaction proceeds similarly, so zinc soaps and cadmium soaps have good initial stability and better initial coloring. For barium and calcium, they almost cannot form covalent bonds with the chlorine atoms on the polymer chain, but instead form unstable dimers due to changes in coordination number. It can be regenerated through other pathways. Therefore, barium soaps and calcium soaps have excellent long-term stability.
(2) Color Stabilization Mechanism
The complexes of zinc soaps and cadmium soaps can complement the color of PVC, while calcium soaps and barium soaps cannot. Due to the color relationship, if the zinc soaps and calcium soaps in the Ca/Zn composite stabilizer are not properly combined, it may cause PVC to discolor.
2.4.3 Organotin
(1) Reaction with HCL
Both Carboxylic Acid Organotins and Thiol Organotins can react with HCL produced during PVC degradation.
(2) Reaction with Unstable Chlorine Atoms
Alkyl tin can react with unstable chlorine atoms, thereby limiting the initiation zone of HCl Elimination and preventing the formation of large conjugated structures. Thiol organotin can also replace unstable chlorine atoms in PVC.
(3) Addition to conjugated double bonds
Tin maleates can easily undergo a “Diene Addition” Reaction with the conjugated double bonds in PVC molecules, resulting in the fixation of the conjugated double bonds and the inhibition of the growth of conjugated chains. The thiol generated from the reaction of thiol organotin with HCl can also undergo addition reactions with conjugated double bonds.
(4) Ability to capture free radicals
When organotin reacts with macromolecular free radicals, it terminates the free radicals and becomes a relatively stable free radical itself.
(5) Decomposition of hydrogen peroxides
Sulfur-containing Organotins have antioxidant properties and can decompose hydrogen peroxides, preventing the formation of new free radicals from the thermal decomposition of hydrogen peroxides and reducing the concentration of free radicals in the system to achieve stabilization.
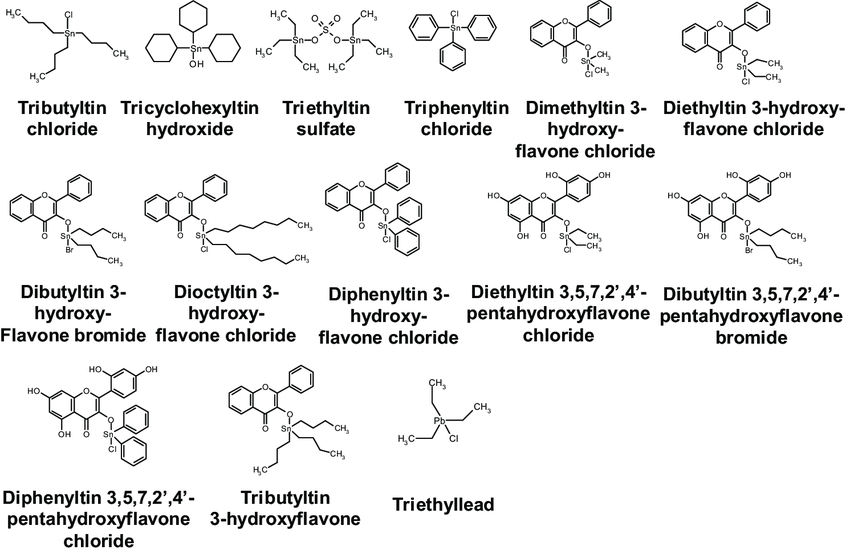
2.4.4 Rare earth stabilizers
Rare earth stabilizers have the ability to form coordination complexes. During the release of HCl in PVC processing, they can absorb a large amount of HCl and stabilize the Cl- in PVC molecules, especially the unstable allylic and tertiary chlorine atoms, thereby stabilizing PVC.
2.4.5 Epoxy Compounds
Epoxy compounds can directly react with HCl released during PVC processing and absorb unstable allylic chlorine. They can also undergo addition reactions with double bonds.
2.4.6 Polyols
The use of polyols in combination with zinc, calcium, cadmium, etc. soaps can significantly reduce HCl elimination rate. This is because the hydroxyl groups of polyols can form complexes with CdCl2, ZnCl2, etc., reducing their catalytic effect on HCL elimination.
2.4.7 β-diketones
In the presence of ZnCl2, β-diketone compounds can replace allylic chlorine in PVC through carbon alkylation, interrupting and shortening the conjugated polyolefins.
2.5 Synergistic Mechanism of Heat Stabilizers
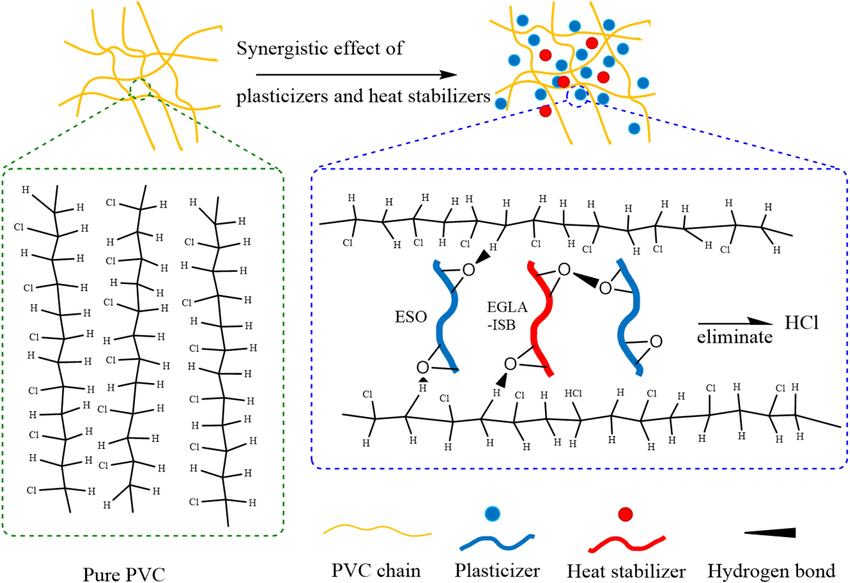
2.5.1 Metal Soaps
According to the active mechanism of metal soaps in preventing the degradation of PVC, they can be classified into two categories: one can only absorb HCl and prevent its catalytic effect on the dehydrochlorination reaction. The most representative examples are barium soaps and calcium soaps. The thermal stability of this type of metal soaps is generally poor, and the initial stability is not good. However, when heated for a long time, the PVC does not change much in stability. The metal chlorides generated during the stabilization process have little catalytic effect on dehydrochlorination. The other type can not only absorb HCl but also react with allyl chloride to stabilize PVC. The most representative examples are zinc soaps and cadmium soaps. This type of metal soaps has good initial coloration, but when heated for a long time, the products change color rapidly. Especially zinc soaps, they are extremely prone to rapid discoloration, resulting in the so-called “zinc burn” phenomenon. This is because the chlorides CdCl2 and ZnCl2 generated during the stabilization process of zinc and cadmium soaps are extremely strong Lewis acids and act as catalysts for dehydrochlorination. Based on the characteristics mentioned above, it is difficult to achieve satisfactory results by using any one type of metal soaps alone. If the highly active cadmium and zinc soaps are used in combination with the less active barium and calcium soaps, both the initial coloration and long-term stability can be improved. For example, when barium soaps are used in combination with cadmium soaps, the cadmium soaps first undergo esterification reactions with allyl chloride in the PVC molecules, generating CdCl2, which then undergoes a metathesis reaction with barium soaps, regenerating cadmium and rendering CdCl2 harmless. The same principle applies to the combination of calcium and zinc soaps, as well as barium and zinc soaps.
2.5.2 Phosphites and Metal Soaps
When phosphites are used in combination with metal soaps, they can react with metal chlorides to inhibit their catalytic effect on dehydrochlorination, thereby improving the thermal stability of the system.
2.5.3 Polyols and Metal Soaps
The combination of polyols and metal soaps can significantly extend the induction period of dehydrochlorination and inhibit resin discoloration. It is generally believed that polyols exert their synergistic effect by complexing with metal chlorides to inhibit their catalytic effect on dehydrochlorination.
2.5.4 β-Diketones and Metal Soaps
β-Diketones can react with PVC through carbon alkylization to stabilize it, but the reaction rate is slow. When used in combination with calcium/zinc systems, the stabilization reaction rate can be greatly increased. Zinc soaps have a high ionization potential and react with allyl chloride to esterify and stabilize PVC. However, the by-product ZnCl₂ is a catalyst for dehydrochlorination and its presence is detrimental. However, ZnCl₂ is also a catalyst for carbon alkylization. The addition of β-diketones takes advantage of this catalytic effect of ZnCl₂ to rapidly carry out the carbon alkylization reaction of allyl chloride. The synergistic effect of β-diketones and barium/zinc is similar.
2.5.5 Rare Earth Stabilizers and Zinc Soaps
Rare earth stabilizers themselves have the effect of displacing allyl chloride, but when used alone, PVC products show yellow discoloration. When used in combination with zinc soaps, the ZnCl₂ generated during the stabilization process of zinc soaps undergoes an exchange reaction with rare earth ions to form less harmful ReCl₃. Additionally, rare earths preferentially react with HCl to form rare earth carboxylates, reducing the catalytic effect of ZnCl₂ on dehydrochlorination. The combination of the two components results in better initial coloring and significantly improved long-term stability.
Conclusion
The above content comprehensively covers the core technical logic of PVC heat stabilizers – from “why stabilization is needed” to “how to choose stabilizers”, and then to “how to enhance the stabilizing effect through synergy”. Each mechanism and conclusion is derived from industry practice and technical accumulation.
As a leading injection molding company specializing in PVC additives, KingStar always aims for “technology empowering production”: Based on the principles described in the text, we have developed a full range of heat stabilizer products including lead salts, metal soaps, organic tin, and rare earths, which can meet special requirements such as transparent products and non-toxic scenarios, and also provide customized solutions for processing problems such as “zinc burning”, “initial coloring”, “long-term weather resistance” and so on.
If you encounter any confusion in optimizing PVC formulations or improving stability, please feel free to contact our technical team(sales@kingstarmold.com) at any time – not only do we provide high-quality additives, but we are also willing to work with you with professional technology to jointly enhance the quality and market competitiveness of PVC products.
Learn more about plasticizers in our related posts about plasticizers:
Key Types of PVC Thermal Stabilizers: Properties, Applications & Selection Tips
Properties of PVC Heat Stabilizers: A Comprehensive Guide