The most fundamental properties of heat stabilizers are heat resistance, weather resistance, and processability. Additionally, from the perspective of PVC product usage, other factors such as transparency, compatibility, mechanical properties, electrical insulation, sulfurization resistance, toxicity, etc. must also be considered. With the development of the heat stabilizer industry, the use of composite heat stabilizers has increased, and they are always used together with many other additives, such as plasticizers and processing modifiers. In such cases, issues such as the impact of other additives on the performance of heat stabilizers need to be considered. Therefore, in this article, as the leading PVC injection molding company in China, we provide some fundamental knowledge to help you master the basic properties of various heat stabilizers, reasonably selecting and using the most suitable heat stabilizers to maximize their positive properties and avoid negative effects.
1. Heat Resistance
The main function of heat stabilizers is to improve PVC’s heat resistance, which is the fundamental reason for using heat stabilizers in PVC processing.
1.1 Concept of Heat Resistance
(1) Initial heat resistance and long-term heat resistance
The initial heat resistance of heat stabilizers refers to the ability that improves the initial coloring of PVC product. The long-term heat resistance refers to the ability of PVC products to maintain their original color at high temperatures for a long time. There is no necessary connection between long-term heat resistance and initial heat resistance. Some heat stabilizers have poor initial heat resistance but good long-term heat resistance, thus called persistent type. Some heat stabilizers have poor long-term heat resistance but good initial heat resistance, thus called short-term type.
(2) Static heat resistance and dynamic heat resistance
Static heat resistance refers to the heat resistance of the sample during the heat treatment under static conditions, which reflects the heat resistance of a simple chemical reaction. There are similar reactions in PVC casting and molding and the processing of plastic products. Dynamic heat resistance refers to the heat resistance of the sample under the action of mechanical shear force, pressure, and heat, which reflects not only the heat resistance of chemical reactions but also the dynamic heat resistance of processing.
1.2 Heat Stability of Various Heat Stabilizers
About dynamic heat resistance, metal soaps have good performance, but there are also poor ones, so they often need to be used in combination. Lead salts have little difference in dynamic and long-term heat resistance, and must be used with lubricants. As for static heat resistance and dynamic heat resistance, various stabilizers have their own characteristics. The stability of metal soaps varies with the type of metal, and alkali metal and alkaline earth metal soaps belong to long-term type, with poor initial stability, such as aluminum, calcium, magnesium, barium, etc. While zinc and cadmium belong to short-term type, with poor long-term stability. Lead soaps are between the two types. The organic acid radicals of metal soaps have no effect on the initial type and long-term type.
Lead salts possess initial coloring phenomenon and belong to long-term type. The acid groups of lead salts have a significant impact on heat resistance. In commonly used lead salt products, sulfite salts have the best performance, followed by sulfate salts, and the least is phosphite salts. Basic lead sulfite has the best heat resistance among lead salts. In organotin compounds, laurate esters have poor initial heat resistance but good long-term heat resistance; maleate esters have good initial coloring and long-term heat resistance. Thiol esters have no initial coloring, but good long-term heat resistance. Antimony-based stabilizers have excellent initial and long-term heat resistance, and their performance is similar to some extent to organotin, but still cannot not as good as organotin. They have good synergistic effects with calcium stearate, epoxides, and phosphoric esters. Rare earth-based stabilizers have both initial and long-term heat resistance. The performance of various single rare earth-based stabilizers is not much different, while the performance of mixed rare earth-based stabilizers is not as good as a single variety. Epoxides, polyols, and phosphoric esters are common long-term type stabilizers. While β-diketone compounds belong to short-term type and have limited effect on improving long-term stability, so they are often used as auxiliary stabilizers.
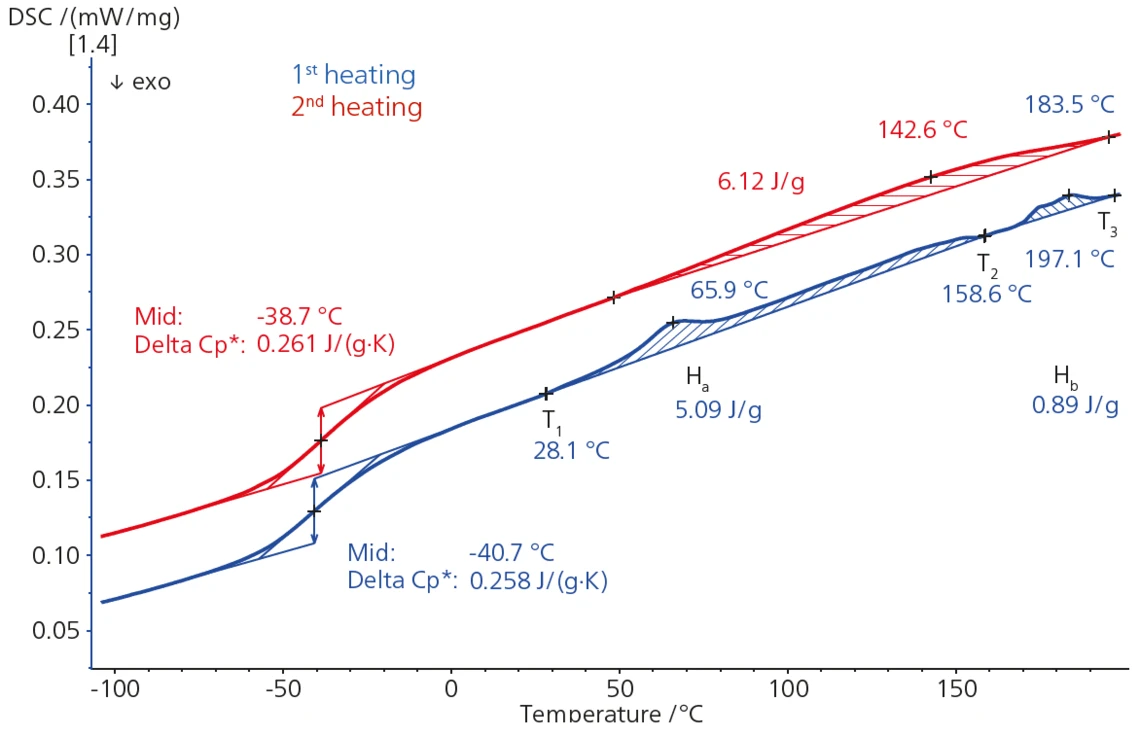
DSC Analysis of Thermal Behavior of Plasticized PVC
1.3 Synergistic Effect of Heat Stabilizers
Heat stability is the most fundamental property of heat stabilizers. A good heat stabilizer should not only have excellent static heat resistance but also excellent dynamic heat resistance; not only should it have excellent initial stability but also excellent long-term stability; not only should it withstand the high temperatures during processing but also withstand the long-term use of plastics. Therefore, different physical properties of heat stabilizers need to be compounded to utilize the synergistic effect of the components and meet different requirements.
(1) The synergistic effect of metal soaps: Alkali metals and alkaline earth metal soaps belong to the long-term type, while zinc and cadmium belong to the initial type. The combined use of these compounds can not only achieve good heat resistance but also good weather resistance and processing properties.
(2) The synergistic effect of metal soaps and β-diketone compounds: The presence of β-diketone compounds can greatly improve the initial coloring. For certain structures of β-diketone compounds, almost no chloride ions can be detected within 30 minutes of degradation.
2. Processing Properties
The influence of heat stabilizers on the processing properties of the prepared PVC materials depends on the amount of the heat stabilizer added and the specific chemical properties of the heat stabilizer system.
(1) Metal Soaps: In metal soaps, lead, cadmium, and zinc soaps have good lubricity and low mixing torque values, and gelation occurs slowly; magnesium, calcium, strontium, and barium soaps have good gelation performance and high mixing torque values. Zinc soaps delay gelation. For the same metal soaps, different organic acid groups result in different processing properties. Aliphatic hydroxy acid soaps simulate the lubricity of aromatic soaps better. In fatty acid groups, as the molecular chain grows, the lubricity also increases accordingly.
(2) Lead Salts: Lead salts have a high relative density and are prone to coagulation. They are difficult to be dispersed evenly during mixing, resulting in poor processing properties, and require the use of both metal soaps and lubricants.
(3) Organic Antimony: Organic antimony stabilizers have poor lubricity and usually require the addition of a large amount of lubricants.
3. Transparency
3.1 Transparency of Various Stabilizers
Generally, organotin stabilizers have the best transparency and are mainly used in transparent products. Among them, tin maleates and thiol tins are the best transparent heat stabilizers. Rare earth stabilizers have a refractive index very close to that of PVC resin, thus also possessing good transparency. In soft transparent products, barium/cadmium/zinc liquid composite stabilizers are mostly used, and barium zinc stabilizers are often used when resistance to sulfur contamination is required, while calcium/zinc metal soaps are often used when non-toxicity is required. Lead salts are opaque and cannot be used in transparent products. The transparency of the stabilizer itself is important, but the compatibility, volatility, processing properties, and processing operations of the stabilizer also affect the transparency of PVC products. For example, if the temperature is too low during extrusion, fog-like phenomena will appear on the surface of the product.
3.2 Opacity Phenomenon and Elimination
1. Whitening Phenomenon: Transparent/clear PVC plastic parts will present a white turbid state after bending, stretching, water immersion, or outdoor exposure. This phenomenon is called whitening. Whitening may be caused by external force causing changes in the density and molecular structure of PVC, or by the action of water, sweating, spray frosting, or various complex factors such as photo degradation and dust adsorption.
(a) Stress Whitening: Stress whitening is a whitening phenomenon in PVC products under mechanical external force. This is because the PVC molecular chains orient along the tensile stress direction, causing density changes, and at the same time, gaps appear between molecules, resulting in light scattering and presenting a white color. Stress whitening is particularly severe when using lead salts. Using organotin stabilizers can reduce whitening. This is because adding a stabilizer with high surface energy can compensate for the gaps caused by molecular orientation and effectively reduce the whitening phenomenon.
(b) Water Immersion Whitening: When transparent PVC products are immersed in water for a period of time, a turbid and opaque phenomenon will occur; and this phenomenon is reversible. When thoroughly dried, the turbid product will regain its original transparency. This phenomenon is caused by the presence of stabilizers that are prone to hydration reactions or decomposition products that are easily hydrated. Some people believe that this phenomenon is caused by the water immersion, causing the stabilizers, plasticizers, etc. to be precipitated from PVC. The water immersion whitening phenomenon is related to the compatibility and water extractability of the stabilizer, so a stabilizer with good compatibility and poor water solubility is more beneficial for preventing water immersion whitening. In actual use, it is found that almost all alkaline earth metal soaps, especially barium and calcium, are prone to water immersion whitening, while formulations containing cadmium soap and zinc soap only occasionally exhibit this phenomenon. Organotin will not exhibit water immersion whitening.
(c) Exposure Whitening: When PVC parts are placed in the air, due to the action of moisture, sulfur dioxide, carbon dioxide, and light in the atmosphere, whitening will also occur. This is related to the compatibility of the stabilizers. Stabilizers with good compatibility rarely exhibit this phenomenon, while stabilizers with poor compatibility, such as lead salts, are prone to the whitening phenomenon caused by exposure to sunlight.
2. Fish eyes and bubbles: When observing transparent or semi-transparent PVC sheets under light, one can find small transparent or semi-transparent round particles similar to fish eyes mixed in the sheets. These are called “fish eyes”. Sometimes, bubbles can also be found in transparent sheets. Both fish eyes and bubbles can affect the transparency of the products. Generally, the causes of fish eyes are mostly in the resin itself. However, if the stabilizer mixture is not uniform, fish eyes can also occur. The generation of bubbles is caused by the volatilization of additives or the imbalance in processing. It can be seen that stabilizers with good dispersion and low volatility are beneficial for reducing fish eyes and bubbles.
4. Weather Resistance
PVC products used outdoors for long-term exposure will not only age by sunlight, but also be affected by wind, snow, rain, dew, day-night alternation, seasonal changes, and air pollution. The aging phenomenon caused by these natural factors is usually called weathering aging. The property of resisting weathering is called weather resistance.
4.1 Weather Resistance of Various Stabilizers
Lead salt-based stabilizers have poor weather resistance. Among them, phosphites have the best weather resistance, sulfites are second, and sulfates are the worst. Among metal soaps, cadmium soaps have excellent weather resistance, zinc soaps, lead soaps, and barium soaps are second, and calcium soaps have poor weather resistance. In composite metal soaps, barium/lead/ cadmium soaps have excellent weather resistance, while calcium/zinc soaps have poor weather resistance. In organotin stabilizers, maleic anhydride has the best weather resistance, lauric acid has the second best, and sulfates are relatively worse. Organic antimony stabilizers have extremely poor weather resistance and require storage in opaque containers. Rare earth stabilizers have anti-photoaging effect, and their weather resistance performance is better than lead-based stabilizers and comparable to organotin.
When different types of stabilizers interact, due to synergistic effects, the weather resistance can be greatly improved. UV shielding agents and UV absorbers can prevent degradation caused by UV rays, and when used within other thermal stabilizer systems, the weather resistance of the complex can be greatly improved. In soft PVC transparent products, the combination of barium/cadmium/epoxy compound/phosphoric ester has the best weather resistance. In this combination, the phosphoric ester has a particularly significant improvement on the weather resistance of the barium/cadmium system, while the epoxy compound mainly improves its thermal stability. In soft non-transparent products, base-type lead salts have good weather resistance. If phosphoric or sulfurous salts are added to the tri-base sulfuric lead salt, its weather resistance can be improved to the same level as the di-base phosphoric ester lead salt. If a stabilizer system of barium/cadmium/epoxy compound/phosphoric ester is used and supplemented with UV shielding agent titanium dioxide, its weather resistance is better than that of the di-base phosphoric ester lead salt. In non-transparent products, base-type lead salts have good weather resistance. Due to the tendency to turn black when used with lead stabilizers under light, this combination is not recommended. Lead stabilizers may also exhibit whitening when aged, and when used with anatase titanium dioxide, the effect is more obvious.
4.2 Test Methods for Weather Resistance
The test methods for weather resistance include natural outdoor large chlorine test, accelerated outdoor atmospheric aging test, and laboratory accelerated aging test.
5. Compatibility
5.1 Incompatibility Phenomenon
Stabilizers tend to disperse in PVC compounds, so if they don’t leach out even after long-term use of the finished products, it indicates that they have good compatibility, which is a preferrable outcome. However, not all stabilizers can be fully compatible with the compounds. In such cases, the stabilizers will move from the interior of the products to the surface and eventually leach out. If the leached-out substances are powdery solid substances, it is called “blooming”; if they are liquid substances, it is called “exudation” (also called “sweating”). Both “blooming ” and “exudation” are due to incompatibility, and their occurrence will affect the surface properties and electrical properties of the products.
The degree of compatibility of PVC depends on many different, independent factors. These factors include the compatibility of each component in the stabilizer formula, the content of each component in the entire PVC formula, and the properties of the components that constitute the entire PVC formula composition. Additionally, chemical reactions between different components during the processing also play a role. “blooming ” and “exudation” are often latent phenomena. Sometimes they can be observed during the processing of the ingredients, while in other cases, it takes a long time to be observed.

Plasticizer Migration Phenomenon in Flooring Product
5.2 Compatibility of Various Stabilizers
Generally speaking, lead salt stabilizers have poor compatibility, but tribasic sulfuric lead sulfate has no tendency to increase or reduce “blooming”.
In metal soaps, types of themselves and types of organic acids both have an impact on “blooming”. Metals with low electronegativity (lower valence) such as barium and calcium have less “blooming”, while metal soaps and aluminum soaps are more prone to it, but the “blooming” of metal soaps is less than that of the corresponding acids. As for organic acids, aromatic acids have less “blooming” than fatty acids, and the longer the carbon chain of the fatty acids, the more “blooming” it shows. Stearic acid soap is prone to “blooming”, followed by lauric acid, then caprylic acid soaps, and butylbenzoic acid soap rarely produces “blooming”. Organotin stabilizers have excellent compatibility, so they generally do not exhibit the “blooming” and “exudation” phenomena that are common in many lead salt stabilizers like barium/cadmium, barium/zinc, and calcium/zinc stabilization systems. The thiol-based organotin has the best performance, followed by lauric acid organotin, and maleic acid organotin is also prone to “blooming”. Phosphites and phosphates have a tendency to increase chances of “blooming”, while epoxides and calcium carbonate have no effect on compatibility.
The surface of the products with exudation, apart from feeling slippery or sticky, often has an unpleasant smell. The exudation substances will also greatly damage the mechanical strength, elongation, chemical resistance, electrical insulation, optical properties, and weather resistance of the products. To prevent “blooming”, when using heat stabilizers prone to “blooming”, adding a small amount of ” blooming inhibitors ” is effective.
6. Plate-out Property
6.1 Plate-out and Plate-out Property
During plastic processing, components such as pigments, lubricants, stabilizers, or plasticizers in the compounding agent disperse and adhere to the metal surfaces of the press roller, mold, etc., gradually forming harmful film layers. This phenomenon is called “plate-out”. Sometimes it is also called “fouling” or “surface coating”. The property of components in the compounding agent causing plate-out during processing is called the plate-out property.
Plate-out often occurs on the cold rollers after extrusion and the post-extrusion rollers, but it can also occur in the mixing machine or extrusion machine. In extrusion processing, plate-out also occurs on the screw, barrel wall, inner wall of the die, and the traction device after the extruder, and the fouling can cause many troubles in the molding operation. When the accumulation is severe, it can make it difficult to peel the part off from the screw, and at the same time, the surface of the product is contaminated. It is generally believed that the incompatibility of raw materials is part of the reason for the occurrence of plate-out, but it is not the only reason. However, in any type of molding processing, changes in various raw materials, such as resins, plasticizers, fillers, lubricants, and stabilizers, as well as changes in processing conditions, all affect the severity of plate-out. By changing the above conditions, it is likely to cause plate-out that originally won’t occur in formulations or processes. Similarly, changing the above conditions may also alleviate or even eliminate the previously severe plate-out phenomenon.
6.2 Plate-out Property of Various Stabilizers
The plate-out properties of various stabilizers vary greatly. Generally speaking, metal soaps have a significant impact on plate-out, while inorganic lead salts and organotin stabilizers do not cause plate-out. Large dosage of rare earth stabilizers may cause plate-out, non-ideal roll-release property and slight adhesion. Among metal soaps, strontium, magnesium, calcium, barium, and cadmium soaps are more prone to plate-out, while zinc and tin are relatively less prone to plate-out. Additionally, the plate-out property of organic acid groups has a certain influence on metal soaps. For the same metal soap, aromatic acid soaps have less plate-out, while fatty acid soaps have more plate-out. At the same time, as the carbon chain of the fatty acid group increases, the corresponding plate-out phenomenon becomes more severe. The plate-out property is related to the dosage of the stabilizer. Within a certain range, as the dosage of the stabilizer increases, plate-out also increases accordingly. However, when the dosage of the stabilizer exceeds a certain value, plate-out will no longer continue to increase. Generally speaking, in actual processing, the higher the processing temperature and the longer the processing time, the more severe the plate-out phenomenon would be.

Plate-out Phenomenon during Injection Molding
6.3 Prevention of plate-out
Although the factors affecting plate-out are very complex, over a long period of processing practice, people have summarized some basic rules of specific measures to prevent plate-out to minimize it to the greatest extent.
(1) Plate-out usually occurs only in PVC formulations that contain both a alkaline earth metal salt and a heavy metal salt. The alkaline earth metal salts may come from fillers, stabilizers, or lubricants (calcium stearate), while the heavy metals may come from pigments (titanium, manganese, lead), stabilizers (cadmium, zinc, lead), or from other sources.
(2) The formation speed of fouling depends on the production speed of PVC products, the percentage of metals in the formula, and the chemical form of the added metals.
(3) Adjusting the concentration of alkaline earth metal salt stabilizers is an effective method to control the occurrence of plate-out. Under the premise of meeting the stability requirements, the dosage should be reduced to the minimum. Partial or complete replacement of alkaline earth metal salts with alkali metals can help reduce plate-out.
(4) The use of stabilizers in combination can reduce plate-out. Barium stearate, calcium stearate, etc. have a high plate-out property. If combined with zinc stearate, cadmium stearate, etc., it can effectively reduce plate-out. Aside from zinc stearate and cadmium stearate, hard acid lead stearate, aluminum stearate, triphenyl phosphate, etc. can also be used.
(5) Adding certain plate-out prevention agents can prevent the plate-out phenomenon. Adding cationic or anionic surfactants often helps reduce or eliminate plate-out; adding zinc-based stabilizers based on stearic acid or zinc stearate, especially in liquid form, can effectively reduce plate-out; adding aluminum oxide, silica gel, triphenyl phosphate ester, etc. can also help inhibiting plate-out.
(6) Organotin stabilizers, regardless of which formula they are used in or which processing method is adopted, will not cause plate-out.
(7) The surface condition of rollers or screws is subtly related with plate-out. Therefore, the roller surface should be kept smooth, and attention should be paid to preventing various foreign substances, corrosive substances, etc. from damaging the surface of rollers or screws. When changing the formula, the processing equipment should be cleaned thoroughly.
(8) The degree of plate-out is related to the moisture in the air. When the moisture is high, it is prone to cause plate-out.
7. Electrical properties
7.1 Electrical insulation and anti-static properties
When designing the formula for insulating materials such as wires and cables, it is required that the materials should not contain ionic materials. The stabilizers in the materials should not only be able to prevent the color darkening caused by PVC degradation, but also ensure that the stabilized products are non-ionic. Stabilizers without this function cannot be used in products requiring electrical performance. During the stabilization process, non-ionized compounds are formed, resulting in non-conductive properties, which is commonly referred to as the electrical insulation property of the stabilizer. Contrary to this situation, when manufacturing products such as flooring and carpets, in order to prevent static electricity accumulation, it is often required that the stabilizer used generates conductive metal chlorides during the stabilization process. This kind of stabilizer is seen as possessing anti-static properties.
7.2 Evaluation of Electrical Properties
Some formulations used as electrical insulating materials must take the electrical properties of the materials into account, including volume resistivity, dielectric strength, dielectric constant, and power factor, etc.

PVC Stabilizer for Wires and Cables
7.3 Electrical Properties of Various Stabilizers
Generally, inorganic salts and metal soaps have good electrical insulation properties and are suitable for use in wire insulation and insulation formulations. Organotin, liquid barium/cadmium composite stabilizers have low electrical insulation properties and can be used in formulations for preventing static electricity.
8. Toxicity
(1) Lead salts
Lead salts have strong toxicity and are categorized into highly toxic stabilizers. People who involve in long-term contact should pay attention to the following points: 1. Use effective dust-proof masks. 2. Rinse mouth with a dilute solution of 3% sodium thiosulfate or 0.5% salt water mixed with menthol. 3. Take a thorough bath. 4. Take vitamin supplements.
(2) Cadmium soap
Cadmium soap is a highly toxic environmental pollutant. If a person consumes more than 300 μg of cadmium per day, there is a risk of osteoporosis.
(3) Barium soap
The toxicity of barium is strong and it cannot be used in non-toxic formulations.
Mastering the strategic use of heat stabilizers and other additives is just one piece of the puzzle in optimizing PVC production. What truly drives efficiency, quality, and cost savings is integrating these technical insights into your unique workflow, whether you’re refining formulations, adjusting processing parameters, or resolving persistent quality issues like “zinc burning” or plate-out.
As a dedicated injection molding company, we deliver end-to-end project solutions tailored to your operational needs. Our team of technical experts works with you to assess your current production lines, identify bottlenecks, and design customized optimization plans: from selecting the right stabilizer systems and fine-tuning dosages to optimizing mixing, extrusion, or molding processes. We prioritize alignment with European and American market standards, ensuring your products meet regulatory requirements while reducing waste, lowering operational costs, and enhancing long-term performance.
Ready to transform your PVC production with data-driven process optimization? Contact us today at sales@kingstarmold.com to discuss your specific challenges, request a free process assessment, or collaborate on a tailored optimization project. Let’s turn technical insights into tangible operational improvements that boost your competitiveness in global markets.
Learn more about plasticizers in our related posts about plasticizers:
Key Types of PVC Thermal Stabilizers: Properties, Applications & Selection Tips
PVC Heat Stabilizers: Classification, Mechanisms & Key Insights for Formulation