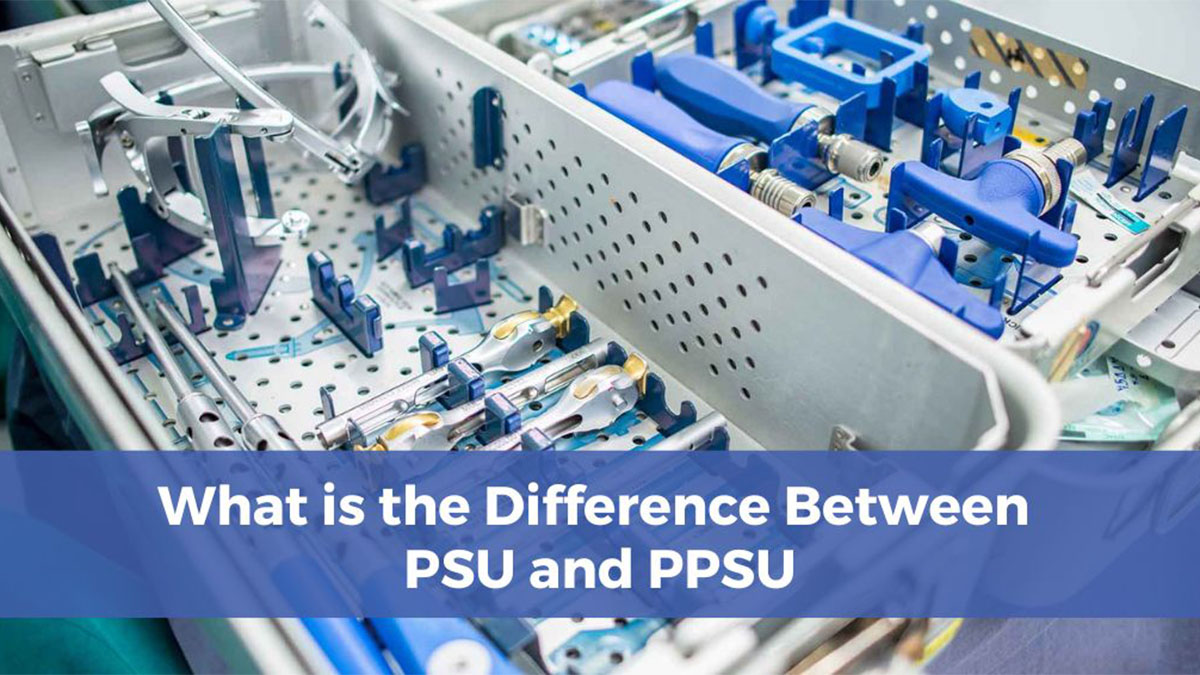
Polyphenylene sulfone (PPSU) and polysulfone (PSU) belong to the sulfone class of amorphous high-performance polymers. With excellent biocompatibility, heat resistance, and chemical stability, they have always been the core injection molding materials in high-end fields such as medical devices and food contact. The two may appear to have similar performance, but there are significant differences in actual processing characteristics, applicable scenarios, cost control, and other aspects. Choosing the right material directly determines the product’s qualification rate, service life, and market competitiveness.
1. What’s the Difference Between PPSU and PSU? Core Material Property Comparison
(1) Heat Resistance & Sterilization Tolerance:
- The glass transition temperature (Tg) of PPSU reaches 220 ℃, the thermal deformation temperature is about 207 ℃, and the long-term continuous use temperature can be stable at 180 ℃. In the medical sterilization scenario, it can withstand steam sterilization at a high temperature of 134 ℃ for more than 1000 cycles without deformation, brittleness.
- The glass transition temperature (Tg) of PSU is 185 ℃, the thermal deformation temperature is 175 ℃, and the upper limit of long-term use temperature is 150 ℃. It is only suitable for low-temperature steam sterilization at 121 ℃, with a tolerance of about 100-200 cycles, and cannot withstand hydrogen peroxide sterilization. Once overheated or sterilized too many cycles, the material is prone to yellowing and cracking, which affects product performance.
(2) Mechanical Properties:
The mechanical properties of the two materials have their own emphasis, suitable for injection molded parts with different structural requirements:
- PSU’s advantage lies in rigidity: its bending modulus is slightly higher than PPSU, and the formed product is less prone to deformation, making it particularly suitable for making handheld medical devices that require precise size maintenance, such as the support parts of surgical forceps and tweezers, as well as the housing frames of medical equipment – if these components bend during use, it will directly affect operational accuracy.
- PPSU’s advantage lies in toughness: its notch impact strength can reach 694 J/m, its tensile elongation is higher, and its resistance to drop and impact is outstanding. Parts that require frequent grip and may collide, such as endoscope handles and dental phone cases, can be significantly reduced in damage rate by using PPSU injection molding.
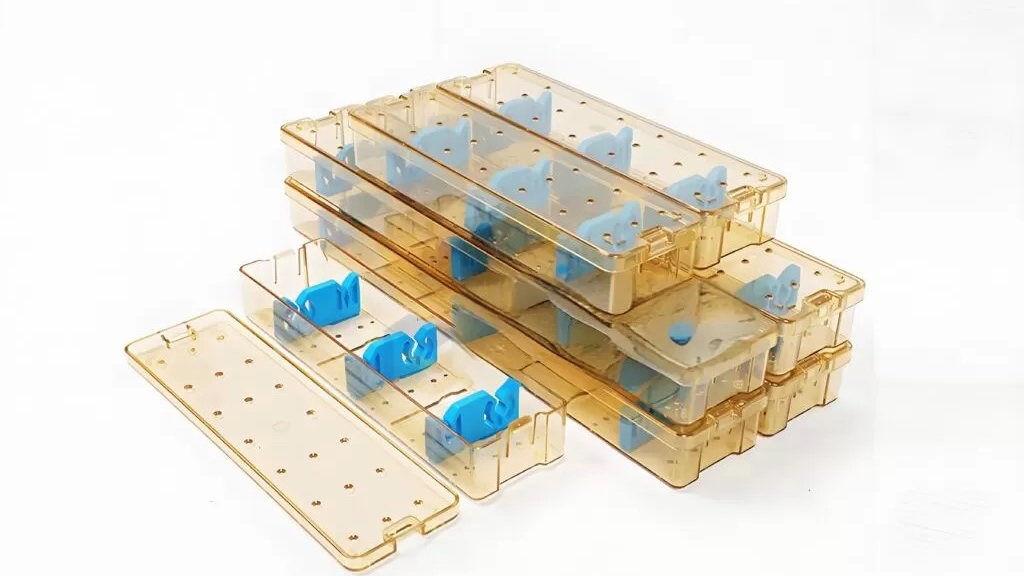
(3) Appearance & Transparency:
In medical devices, transparency is often related to usage safety, which is also one of the core competitiveness of PSU:
- PSU is light colorless or slightly yellow in color, with extremely high transparency. It can clearly observe the color, flow rate, and liquid level of the internal fluid. It is the preferred material for products such as hemodialyzers, infusion sets, and filter membranes. In addition, it can also be dyed into a color encoder device for quick identification during surgery.
- PPSU naturally has an amber color and slightly lower transparency. Although it can be improved through process optimization, it is difficult to achieve the transparency effect of PSU. However, its resistance to yellowing is better, and after long-term repeated sterilization, the appearance changes are much smaller than PSU.
(4) Chemical Stability and Cost:
- Chemical Resistance: Both can tolerate most disinfectants and acid-base solutions in medical environments. But PPSU has better solvent resistance and is less prone to swelling and cracking when in contact with common medical reagents such as alcohol, iodine, and acetone. PSU may experience performance degradation after long-term exposure to strong solvent.
- Cost Difference: PPSU contains a phenyl ring in its molecular structure, and the synthesis process is more complex. The raw material price is about 30% -50% higher than PSU. However, from the perspective of full lifecycle cost, PPSU can actually reduce the cost of frequent replacement of parts due to its longer service life and more sterilization cycles.
(5) Biocompatibility:
Both have passed medical grade certifications such as USP Class VI and ISO 10993. The basic grade are suitable for devices that come into short-term (≤ 24 hours) contact with human body fluids and tissues. Special modified grades (such as PSU’s Eviva series and PPSU’s Zeniva series) can also be used for long-term implantable devices, such as artificial heart valves and auxiliary components for orthopedic implants, to meet the compliance requirements of different medical scenarios.
Key Performance Comparison Table
| Performance Indicator | PPSU (polyphenylene sulfone) | PSU (Polysulfone) |
|---|---|---|
| Glass Transition Temp. (Tg) | 220°C | 185°C |
| Steam Sterilization Tolerance | 134°C, 1000+ cycles; Compatible with hydrogen peroxide sterilization at 121 ℃, 100-200 cycles; | Not compatible with hydrogen peroxide sterilization |
| Mechanical Advantage | high toughness, impact resistance, drop resistance | High rigidity, dimensional stability, bending resistance |
| Transparency | Amber, medium transparency; Excellent yellowing resistance | Pale, high transparency; Ideal for fluid monitoring |
| Solvent Resistance | Excellent, resists most medical reagents | Good; avoid long-term contact with strong solvents |
| Cost Level | Higher; lower total lifecycle cost | Relatively economical; cost-effective |
| Typical Certifications | USP Class VI, ISO 10993; Zeniva grades support long-term implants | USP Class VI, ISO 10993; Eviva grades support long-term implants |
2. Core Differences in Injection Molding Processes for PSU and PPSU
Both PSU and PPSU belong to high-temperature engineering plastics, and the threshold for injection molding processing is much higher than that of ordinary plastics (such as PC and ABS). Minor differences in process parameters can directly affect product quality.
(1) Raw Material Drying:
Both materials have hygroscopicity, and insufficient drying can cause bubbles, splay marks, and even affect biocompatibility in injection molded parts:
- PPSU Drying Requirements: It needs to be dried at 150-177 ℃ for 4-6 hours, with a target moisture content controlled below 0.02%. It is recommended to use a dehumidification dryer with a dew point controlled below -40 ℃. Incomplete drying of PPSU can easily lead to internal pores after injection molding, reducing the product’s pressure resistance.
- PSU Drying Requirements: drying temperature of 135-163 ℃, drying time of 4 hours, and moisture content of less than 0.05%. Compared to PPSU, the drying difficulty of PSU is slightly lower, but it cannot be omitted – the transparency of uncured PSU injection molded parts will significantly decrease, and white mist like defects will appear on the surface.

(2) Molding Temperature:
The molding temperature of both exceeds 300 ℃, which is difficult for ordinary injection molding machines to meet the requirements:
- PPSU Barrel Temperature: It is recommended to set it at 330-360 ℃, and mold temperature at 120-140 ℃. Low temperature of the material barrel can lead to insufficient plasticization, resulting in weld lines and decreased mechanical properties; If the temperature is too high, the material will degrade, resulting in yellowing and brittleness.
- PSU Barrel Temperature: It is recommended to set it at 320-350 ℃ and mold temperature at 100-120 ℃. The melt viscosity of PSU is slightly lower than that of PPSU, and the screw speed can be appropriately reduced to avoid material decomposition caused by shear overheating.
Equipment Requirements: High temperature modified injection molding machines must be selected, and the maximum temperature of the barrel must reach 400 ℃ or above, with temperature control accuracy within ± 1 ℃. Many ordinary injection molding machines in small and medium-sized factories (with a maximum temperature of 350 ℃) are prone to problems such as insufficient plasticization and short shots.
(3) Mold Design & Material Selection:
- Mold Material Selection: Don’t use low cost pre hardened steel. Use high temperature resistant quenched steel such as H13 and S136. Otherwise, the mold will deform and wear at long-term working temperatures above 120 ℃, resulting in increasing product size deviation.
- Mold Structure Optimization: The flowability of both materials is not as good as that of ordinary plastics. Mold gates should be designed larger and shorter, and a hot runner system should be prioritized to reduce melt flow resistance. It is necessary to design a gradient wall thickness to avoid stress concentration at sudden changes in wall thickness – a main cause of cracking in injection molded parts in the later stage.
(4) Difficulties and Solutions in Process Control
| Processing Challenge | Common Causes | Solutions |
|---|---|---|
| Splay marks/Bubbles on surface | Insufficient material drying; Too high barrel temperature | Extend the drying time and reduce the temperature of the material barrel by 5-10 ℃; Check the dew point of the dehumidifier dryer |
| Clear weld marks and poor mechanical properties | Low melt temperature; Low mold temperature; Unreasonable gate position | Raise the temperature of the material barrel and mold; Optimize the gate position and add exhaust slots |
| Unstable part dimensions | Large mold temperature fluctuations; Improper pressure holding parameters | Adopting a mold temperature machine for precise temperature control; Extend the holding time and set the holding pressure in sections |
| Material degradation/yellowing | The retention time of the material cylinder is too long; The screw speed is too fast | Control shot size to avoid residence time exceeding 20 minutes; Reduce screw rotation speed |
3. How to Choose Between PPSU and PSU? Typical Application Scenario Explained
The key to choosing PPSU or PSU is to consider the product’s usage scenarios and performance requirements, rather than blindly pursuing high-end materials.
(1) Scenarios Where PPSU Injection Molding is Preferred:
- PPSU is most suitable for medical equipment that requires frequent high-temperature sterilization, such as surgical instrument trays, endoscope accessories, dental phone cases, and dialyzer core components. Its resistance to repeated sterilization can greatly extend the service life of the product.
- For components such as the casing of portable medical devices and the wire sheath of implantable devices that require high toughness and drop resistance, choosing PPSU is definitely the right choice. It has strong anti drop ability and can prevent equipment from being damaged or bumped during transportation and use.
- For products that require contact with strong solvents or special sterilization methods, such as endoscopes and chemical reagent detectors that require hydrogen peroxide sterilization, PSU cannot be used. If you insist on using it, it can easily lead to product malfunctions.
- For pats and products for food processing and baby product industries, such as baby feeding products and infant bottles.
(2) Scenarios Where PSU Injection Molding is Preferred:
- For devices such as blood oxygenators, infusion sets, filter membranes, and reagent bottles that require high transparency for observing fluids, choosing PSU is definitely the right choice. Its high transparency allows medical staff to clearly see the internal medium, making it more reassuring to use.
- Surgical forceps, forceps holders, and medical equipment housing frames require high rigidity but do not require frequent sterilization, so PSU is very suitable. It has strong rigidity, can stabilize the shape of the product, and has lower cost, suitable for large-scale production.
- If the project budget is limited and you want to choose materials with high cost-effectiveness, such as reusable medical consumables and laboratory utensils, PSU can meet the basic performance requirements and help save a lot of procurement costs.

4. Professional Injection Molding Support
As a technology-based custom manufacturing company deeply engaged in high-end processes such as IMD/IML and two-shot injection molding, KingStar has created a full process solution from material selection to batch delivery for medical grade high-performance plastics such as PSU and PPSU.
- Material & Equipment Assurance: Cooperate with globally renowned material suppliers to provide medical-grade PPSU/PSU raw materials that comply with USP Class VI certification. Equipped with multiple high-temperature modified injection molding machines (with a maximum barrel temperature of 400 ℃) and precision desiccant drying systems to ensure stable material processing performance.
- Technological Advantages: With over ten years of experience in high-temperature engineering plastic processing, we can adjust mold design and injection parameters according to product structure, and overcome pain points such as weld lines, bubbles, and unstable dimensions. We also have complete mastery of the IMD/IML mold interior decoration process, which can achieve the integrated molding of complex appearance and function.
- Compliance & Quality Control: Establish a clean production workshop that complies with medical industry standards, and implement the ISO 13485 quality management system. Each batch of products comes with a complete material certification report (COA) and process parameter record, supporting the registration and declaration of medical equipment.
- Global ODM/OEM Services: Targeting global brands and buyers, we provide customized services from product design, mold development to mass production. Whether it’s high-precision medical components or high-end food contact products, we can accurately match their needs.
If you are facing the challenge of selecting PSU/PPSU injection molded components or finding a reliable custom manufacturer, please feel free to contact the our team at sales@kingstarmold.comor leave online message. We will provide free suggestions and process evaluation based on your product requirements, to help your product enter mass production as soon as possible.