If you are engaged in an industry related to the manufacturing of plastic products (injection molding, 3D printing, etc.), then you are likely to have encountered a common problem: many thermoplastic materials have a glass transition temperature (Tg) higher than room temperature, which makes them brittle and hard, and even difficult to process, let alone ensuring product performance.
You may have heard of a concept – plasticizers, specifically designed to solve this core problem, transforming ordinary production raw materials into ones suitable for production, or one step further: improving the performance of the final product. But what exactly is a plasticizer? How does it change the properties of polymers? When choosing a plasticizer for your application, what factors should you consider? This guide will provide you with all the information you need to know about plasticizers, including core concepts, mechanism of action, and actual requirements.
1. Overview: Fundamentals of Plasticizers
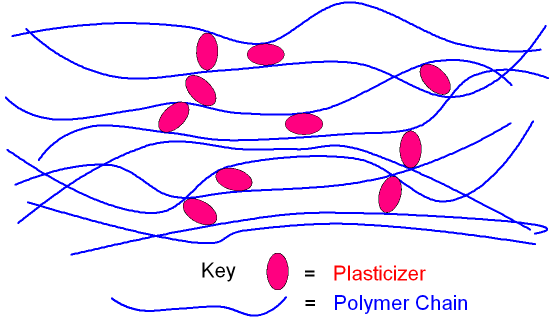
1.1 What Is a Plasticizer? Core Concepts & Key Functions
The glass transition temperature (Tg) of some commonly used thermoplastic polymers is above room temperature. Below the Tg, the polymers exhibit a brittle state similar to that of glass; while above the Tg, they show greater resilience, flexibility, and impact strength. So, to make the polymers possess practical value, their Tg must be lower than their service temperature. Plasticizers, as a type of additive, are introduced into polymers to solve this problem. Plasticizers are substances with relatively low volatility. When added to plastics, they can reduce the elastic modulus and glass transition temperature (Tg) of the plastic, thus provide appropriate flexibility at room temperature, while reducing its melting viscosity at high temperatures to facilitate processing. Broadly speaking, any substance that can mix uniformly with resin, meets either of the following two conditions, and is capable of altering certain physical properties of polymers may be referred to as a plasticizer: it either does not undergo chemical reactions with the resin and remains stable during molding and processing, or it reacts chemically with the resin but remains in polymer products for the long term.
The interaction between polymers and plasticizers can be roughly regarded as the following two ways:
(1) The cancellation of dipole-dipole interactions between resin molecules weakens the intermolecular attraction of resin;
(2) Through a simple dilution effect, the distance (free volume) between polymer molecules is reduced, forming a certain space. Therefore, the flexibility of the plastic sheet material has been improved, and the impact strength of the formed products has also been enhanced.
So, it can be said that the main function of the plasticizer is to weaken the secondary bonds between polymer molecules, namely van der Waals forces (VDW), thereby increasing the fluidity of the polymer molecular chains, that is, improving the plasticity of the polymer.
| DOP Dosage (Phr) | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
|---|---|---|---|---|---|---|---|---|---|
| Shore Hardness (Duro, 25oC) | 96C | 86C | 95A | 88A | 81A | 75A | 69A | 63A | 57A |
| Resin Used: P=1450 |
| P Value | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shore A | 100 | 98 | 96 | 94 | 89 | 87 | 85 | 83 | 80 | 77 | 75 | 73 | 70 | 67 | 64 | 62 | 56 |
1.2 Key Requirements for High-Performance Plasticizers (for Industrial Use)
Not all plasticizers are suitable for every application, and for industrial use, it demands strict performance standards. Here are the 13 core requirements a quality plasticizer should meet:
(1) High plasticizing efficiency, achieving high plasticization effect with a small amount of addition.
(2) They need to be compatible with the resin. If the compatibility is insufficient, the plasticizers will separate from the resin, resulting in phenomena such as exudation and sweating.
(3) They should have low volatility, which can reduce the loss of volatile substances during the molding process and storage, thereby minimizing the impact on the product performance to the greatest extent.
(4) Good durability. They should be resistant to water, oil, and organic solvent extraction. The extraction refers to the phenomenon of plasticizers moving into the liquid medium when plastic products come into contact with solvents or cleaning solutions.
(5) Good migration resistance, which means the plasticizer shouldn’t easily migrate from the plasticized product to another plastic product in contact (including different types and dosages of plasticizers).
(6) Good cold resistance. They should maintain good flexibility at low temperatures.
(7) Good heat resistance. They should stay stable at processing and usage temperatures at higher temperatures.
(8) Non-toxic, odorless, tasteless, and colorless.
(9) Good resistance to mold.
(10) Flame retardant.
(11) Good electrical insulation.
(12) Resistant to contamination and chemical erosion.
(13) Low price.
Not a single plasticizer can fully meet all these requirements. When using, one can only choose products with superior comprehensive performance based on the requirements of the product, the performance of the plasticizer, and market conditions. Cost-effectiveness is often a criterion for choosing additives. The higher the cost-effectiveness, the more advantageous the product is.
2. Mechanism of Action: How Do Plasticizers Modify Polymers?
Understanding how plasticizers work is key to selecting the right one for your polymers. Below is a detailed breakdown of the main influencing factors, core mechanisms, and step-by-step process.
2.1 Main Factors Affecting Plasticization
Two factors directly determine how well a plasticizer works: intermolecular forces of polymers and polymer crystallinity.
2.1.1 Intermolecular Forces of Polymers
When plasticizers are added to polymers, the intermolecular interactions between the plasticizer molecules and the interactions between the plasticizer molecules and the polymer molecules will have a significant impact on the plasticizing effect. These interactions mainly include van der Waals forces and hydrogen bonds. Among them, Van der Waals forces can be further divided into dispersion forces, induction forces and orientation forces.
(1) Van der Waals Forces: Dispersion forces exist between all polar or non-polar molecules; they result from the interaction of tiny instantaneous dipoles, causing adjacent dipoles to be in a state of adjacent opposite poles and thus generating an attractive force. When a molecule with a permanent dipole induces an induced dipole in an adjacent non-polar molecule, the molecular attraction between the induced dipole and the permanent dipole is called an induction force. Induction forces are particularly strong for aromatic compounds. The plasticization of polystyrene by low-molecular-weight esters is mainly due to induction forces.
When polar molecules approach each other, due to the orientation of permanent dipoles, an intermolecular force is generated, commonly known as orientation force. The interaction between ester plasticizers and PVC is a typical example.
(2) Hydrogen Bonds: Molecules containing -OH or -NH groups, such as Polyamide (PA) and Polyvinyl Alcohol (PVA), can form hydrogen bonds between their molecules. Hydrogen bonds are relatively strong interaction bonds, and their presence hinders the insertion of plasticizer molecules between polymer molecules. The denser the distribution of hydrogen bonds along the polymer chain, the more difficult it is for plasticizer molecules to insert. Therefore, the plasticizer needs to generate a similar strong interaction with the polymer molecules. As the temperature rises, the thermal motion of molecules hinders the orientation of polymer molecules, and the effect of hydrogen bonds weakens accordingly.
2.1.2 Crystallinity of Polymers
Under appropriate conditions, the molecular chains of stereoregular polymers can crystallize. It is much more difficult for the molecules of plasticizers to insert into the crystalline regions than into the amorphous regions because the free space between polymer chains in the crystalline regions is the smallest. If the molecules of a plasticizer can only insert into the amorphous regions of semicrystalline polymers, such a plasticizer is categorized as a non-solvent plasticizer. If the molecules of a plasticizer can insert not only into the amorphous regions of polymers but also into their crystalline regions, such a plasticizer is a solvent plasticizer—also known as a primary plasticizer.
2.2 Core Mechanisms of How Plasticizers Work
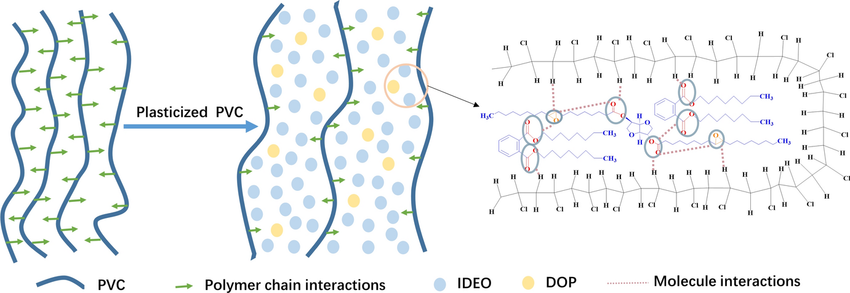
*PVC Plasticizing Mechanism Illustration
2.2.1 Lubrication Theory
The lubrication theory holds that the resistance of resins to deformation (i.e., rigidity) is due to the frictional forces between molecules. Plasticizers act as lubricants, promoting the motion between macromolecules or molecular chains. Plasticizers only reduce the intermolecular forces, thus only causing partial plasticization.
2.2.2 Gel Theory
The gel theory suggests that the resistance of polymers to deformation is due to the presence of a three-dimensional honeycomb structure or gel within them. This gel is formed by the adhesion between polymer molecular chains to varying degrees. Since the adsorption points of molecules are often concentrated in one area, the honeycombs in soft or hard plastics are very small. These honeycombs have very little elasticity and are difficult to deform through internal movement of the object. When plasticizers enter the resin, they generate many adsorption points along the polymer chains, relaxing and breaking the original attractive forces through new adsorption, and replacing the gravitational centers within the polymer molecules, making the molecules more mobile.
2.2.3 Solvation Theory
Based on colloid chemistry. The solvating and swelling ability of plasticizers depends on three types of intermolecular forces: plasticizer/plasticizer, plasticizer/polymer, and polymer/polymer. Plasticizers should be small molecules with a certain attraction to polymer molecules, but this force should be less than the force between polymer/polymer. The lower the force between plasticizer/plasticizer, the more effective the plasticizer, but not too low, otherwise they are prone to volatilization.
2.2.4 Polarity Theory
The polarity theory holds that there must be a good balance between plasticizer molecules, polymer molecules, and the interactions between plasticizer and polymer molecules to ensure gel stability. Therefore, a plasticizer must contain one or more polar or non-polar groups that are polarity-matched with the specific polymer, which is related to the polymer crystallinity mentioned earlier.
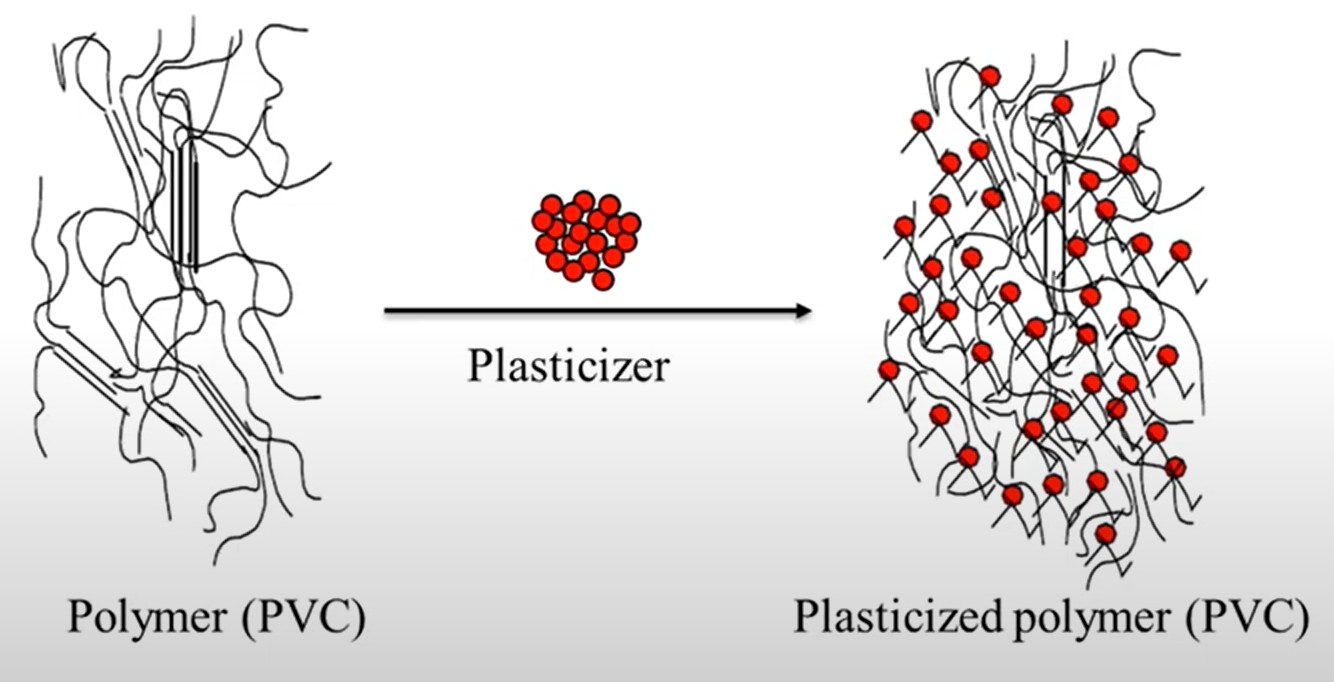
*PVC Structural Differences Before and After Adding Plasticizer
2.3 Step-by-Step Guide to the Plasticization Process
Plasticization process is generally divided into the following steps:
a. Wetting and surface adsorption
The plasticizer molecules enter and fill the resin pores.
b. Surface dissolution
The plasticizer penetrates into the resin particles slowly, especially at low temperatures. It is generally believed that the plasticizer first dissolute and swell the molecules on the polymer surface, and when there are ccolloidal residue from suspension polymerization on the polymer surface, it can prolong the induction stage.
c. Adsorption effect
The resin particles swell gradually from the outside to the inside, generating strong internal stress, which is manifested as a reduction in the total volume of the resin and the plasticizer.
d. Liberation of polar groups
The plasticizer is incorporated into the resin and locally alters its internal structure. Many specific functional groups are dissolved, resulting in a higher dielectric constant after the plasticizer is adsorbed compared to the initial mixture. This process is affected by temperature and activation energy.
e. Structural destruction
The plasticizer in the dry blend exists in the form of molecular aggregates between the polymers or chain segments. When the system is subjected to high energy such as heating to 160 – 180°C or roll milling, the polymer structure will be destroyed, and the plasticizer will penetrate into the molecular aggregates of the polymer.
f. Structural reconstruction
After the mixture of the plasticizer and the polymer is heated to a flowing state and plasticized, and then cooled, a structure different from the original polymer will be formed. This structure exhibits higher toughness, but the formation of the structure often requires a period of time. For example, when using DOP as a plasticizer, it takes one day to reach the maximum hardness, while using medium-molecular-weight polyester, it takes one week.
Conclusion
Plasticizers are indispensable additives for turning brittle thermoplastics into flexible, usable products, from soft PVC pipes to durable rubber seals. By understanding what plasticizers are, their core requirements, how they interact with polymers (via intermolecular forces and crystallinity), and the step-by-step plasticization process, you can make more informed choices for your industrial applications.
Do you have questions about selecting a plasticizer for a specific polymer? Or need help interpreting hardness vs. plasticizer dosage data? Contact KingStar by filling out a form on our contact page or email us at: sales@kingstarmold.com.
Learn more about plasticizers in our related posts about plasticizers:
Plasticizer Performance and Evaluation: PVC Application Focus
PVC Plasticizers: Overview & Selection Tips for PVC Applications



