As a leading custom manufacturing company having tens of years’ experiences in plastic injection molding, I’ve always believed that “choosing the right plasticizer is half the battle.” Whether it’s the “substandard hardness/softness” encountered when adjusting formulas in the workshop, or customer feedback like “product cracking at low temperatures” or “sticking after long-term use,” most of these issues can be traced back to an insufficient understanding of plasticizer performance.
Plasticizers are not just simple “softeners”—every property they have, from basic plasticizing efficiency to durability (which affects product lifespan), processability (which adapts to manufacturing), and even toxicity (which relates to safety), directly dictates the quality and application scenarios of PVC products. For example, when producing food-contact PVC films, we must prioritize plasticizers with good hygienic properties like DOA; when making hoses for outdoor use in northern China, cold resistance and weather resistance need to take center stage. This content is compiled from my years of hands-on experience and literature research, breaking down the 10 core performance dimensions of plasticizers. It covers everything from principles to evaluation indicators, and practical influencing factors—all explained in a down-to-earth way. Whether you’re a new student entering the field, a practitioner debugging formulas on the front line, or a researcher in related areas, you’ll find useful references here. After all, in our industry, once you grasp the basic principles, you’ll be better equipped to solve problems when they arise.
I. Plasticizing Efficiency
Plasticizers mostly function by lowering the softening temperature of polymers, which is achieved by reducing the intermolecular forces between polymer molecules first to increase the mobility of polymer chains. This is the fundamental performance of plasticizers. How efficient different plasticizers are when plasticizing vary, and plasticizing efficiency is introduced to evaluate their performance. Plasticizing efficiency can be understood as the amount of plasticizer used to achieve a certain degree of softness in the resin. It is a relative value that can be used to compare the plasticizing effect of plasticizers.
In practical applications, a reduction in glass transition temperature and modulus is commonly seen when a polymer plasticizes. Therefore, the plasticizing efficiency of plasticizers is often represented by glass transition temperature and modulus.
1. Glass transition temperature
The glass transition temperature measures the mobility of polymer molecular chains, driven by plasticizers which caused movement between these chains. Polymers are soft above the glass transition temperature, and grow hard when the temperature dropped below it. It can be concluded when the addition amount equals, lower the glass transition temperature, better the plasticizing effect of different plasticizers.
Usually, the glass transition temperature of PVC is around 80oC, but with the addition of 10PHR DOP, it significantly decreases by 20-25oC. If 50PHR DOP is added, it would decrease further to -60oC
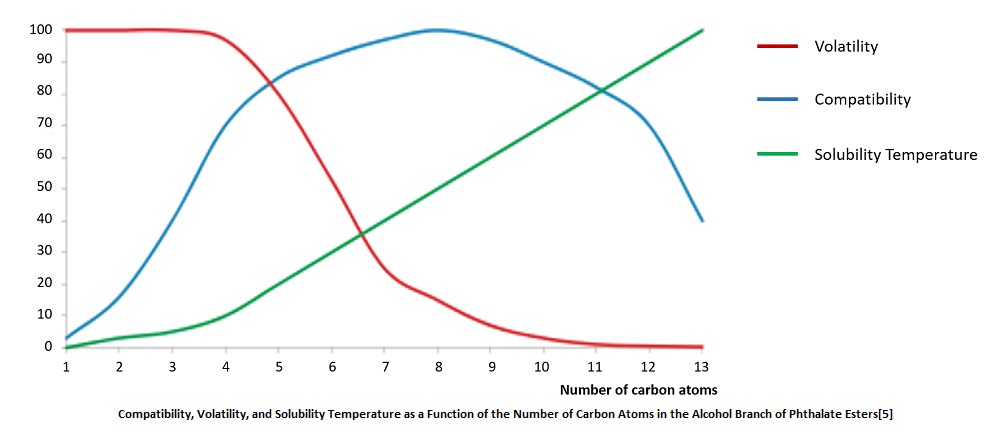
*Data visualized in the chart is adapted from Recent Attempts in the Design of Efficient PVC Plasticizers with Reduced Migration on MDPI, reflecting plasticizers’ glass transition temperature differences and plasticizing efficiency variations.
2. Modulus
Besides the reduction in glass transition temperature, the plasticizing efficiency can also be expressed by some mechanical properties related to temperature dependent, such as modulus and damping. The modulus representation is objective, but quite complex, thus will not be discussed further here.
3. Factors affecting plasticizing efficiency
Plasticizers’ chemical structure and physical properties are all in relation to their plasticizing efficiency, mainly manifested in the following aspects:
(1) The molecular weight of plasticizers affects their plasticizing efficiency. Plasticizers with low molecular weight exhibit good plasticizing efficiency.
(2) When the molecular weight equals, plasticizers with more polar groups or cyclic structures within the molecule would fail in plasticizing efficiency.
(3) The plasticizing efficiency of plasticizers with branched alkyl structures is lower than that of corresponding straight chain alkyl plasticizers.
(4) In ester plasticizers, as the alkyl chain length increases, the plasticizing efficiency decreases.
(5) In ester plasticizers, the alkyl portion is replaced by an aryl group, resulting in a decrease in plasticizing efficiency.
(6) Introducing ether chains into alkyl carbon chains in ester plasticizers enables improved plasticizing efficiency.
(7) Introducing chlorine substituents into alkyl or aryl groups reduces plasticizing efficiency.
(8) Plasticizers’ viscosity is related to their plasticizing efficiency. The equivalent amount of plasticizer increases with its viscosity.
II. Compatibility
Compatibility with resins is one of the fundamental properties for plasticizers. It’s simply impossible to address plasticizing efficiency if plasticizers are incompatible with resins. Tough plasticizers may dissolve some of the resin, but most of it infiltrates between polymer chains to cause swelling, so plasticizers can be simply regarded as solvents. The compatibility of plasticizers can be characterized by a variety of parameters, including solubility parameters, interaction parameters, intrinsic viscosity, dielectric constant, etc.
The structure of polymer and plasticizer have a significant impact on compatibility. A good compatibility comes from similar structures between resins and plasticizers. Phthalates with alkyl carbon atoms ranging from 4 to 10, used as the main plasticizer, have good compatibility with PVC. However, as the number of alkyl carbon atoms further increases, their compatibility rapidly decreases. Therefore, the number of alkyl carbon atoms in the phthalate plasticizers used usually does not exceed 13. Among ester plasticizers, those with amyl as the alkyl group have the best compatibility.
Among epoxy ester plasticizers, polyol esters have better compatibility than monoesters; polyester plasticizers have relatively poor compatibility due to their large molecular weight, which needs to be compensated by intense mechanical mixing at a higher temperature. Although chlorinated paraffins have strong polarity, they still exhibit blooming when used alone and can only be used as secondary plasticizers. In addition, plasticizers with a cyclic structure have better compatibility than those with an aliphatic chain hydrocarbon structure; plasticizers with a branched structure have better compatibility than those with a straight-chain structure.
III. Processing capability
Choosing different plasticizers will significantly affect the processing performance of resin. Therefore, when choosing plasticizers, the processibility of its base must be considered. Being closely related, decent compatibility often comes with good processability for a specific plasticizer. Of course, other performance parameters should also be taken for consideration, such as viscosity, flash point, etc.
The processibility can be exhibited by the gelation rate, gelation temperature, fish eye spot disappearance rate and other parameters. With a large molecular weight, phthalates exhibit a slow gelation rate and a poor compatibility, which reflects the poor processibility of these plasticizers. Also, both stabilizers and lubricants can significantly affect the processability of the compound. If poorly compatible lubricants and large amounts of stabilizers are being applied, the compound’s processibility must be considered to prevent the plasticizers from leaking.
When using lubricants with poor compatibility and large amounts of stabilizers, it is necessary to fully consider the workability of the mixture to prevent the leakage of plasticizers.
IV. Cold resistance
Actually, when we talk about the cold resistance of plasticizers, it actually refers to the cold resistance performance of the plasticized product. Several indicators could reflect this performance, such as low-temperature brittleness temperature and low-temperature flexibility. A common indicator, low-temperature efficiency of the plasticizer, is evaluated by how much glass transition temperature decreases when 1% more mole fraction plasticizer is added to the PVC resin. The low temperature resistance of plasticizers depends on multiple properties. On one hand, the performance is determined by the plasticizer’s structure, including chain length, branching situation, functional groups, types, and quantities. On the other hand, it depends on the polarity effect and isolation effect of the plasticizer entering the polymer chains, as well as the activation energy of the plasticizer itself. The viscosity swells up, leading to higher flowing activation energy, therefore worsening its cold resistance.
Meanwhile, plasticizers’ compatibility is inversely related to cold resistance, for example, binary lipids is mainly composed of linear methylene groups have good cold resistance. Phthalates containing longer straight chains generally exhibit decent cold resistance, but as the alkyl chain increases, the flexibility of the molecular chain starts to decrease, causing plasticizer’s cold resistance to drop. Therefore, as the main plasticizer, straight chain alcohol phthalates exhibit decent cold resistance. When a cyclic structure exists inside the plasticizer’s molecule, its cold resistance will become more significant. At present, aliphatic diesters are mainly adopted as the main cold resistant plasticizers (DOA, DOS, DOZ, etc.).
V. Stability
Plasticizers themselves are excellently chemical stable, Freeing people from worrying about them being affected by light or heat during storage. However, the type and concentration of plasticizers have a significant impact on the thermal stability of polymer complexes.
1. Storage Stability
Storage stability reflects the inherent performance of plasticizers themselves, which can be reduced by the presence of impurities.
2. Thermal Stability
Thermal stability refers to the stability of plastic products when exposed to heat during processing and application. The thermal stability of a plasticizer is directly related to its structure. Beyond the plasticizer’s structure, the following factors also affect thermal stability:
(1) The purity of the plasticizer has a significant impact on thermal stability—the higher the purity, the better the thermal stability.
(2) The lubricity of the plasticizer influences its thermal stability; plasticizers with strong lubricity can significantly improve dynamic thermal stability.
(3) The volatility of the plasticizer affects its dynamic thermal stability; plasticizers with high volatility will significantly reduce thermal stability.
(4) Hydrogen chloride (HCl), a decomposition product of polyvinyl chloride (PVC), acts as a catalyst for the thermal decomposition of carboxylate esters, leading to a decrease in the plasticizer’s own thermal stability.
3. Antioxidant Stability
The antioxidant stability of a plasticizer is related to its own structure. After oxidation of the plasticizer, the change is reflected by an increase in acid value.
4. Light Stability
The light stability of a plasticizer refers to its ability to resist photo-oxidative aging, also known as weather resistance. Generally, plasticizers with excellent cold resistance have poor weather resistance. Epoxy compounds can greatly improve the weather resistance of PVC.
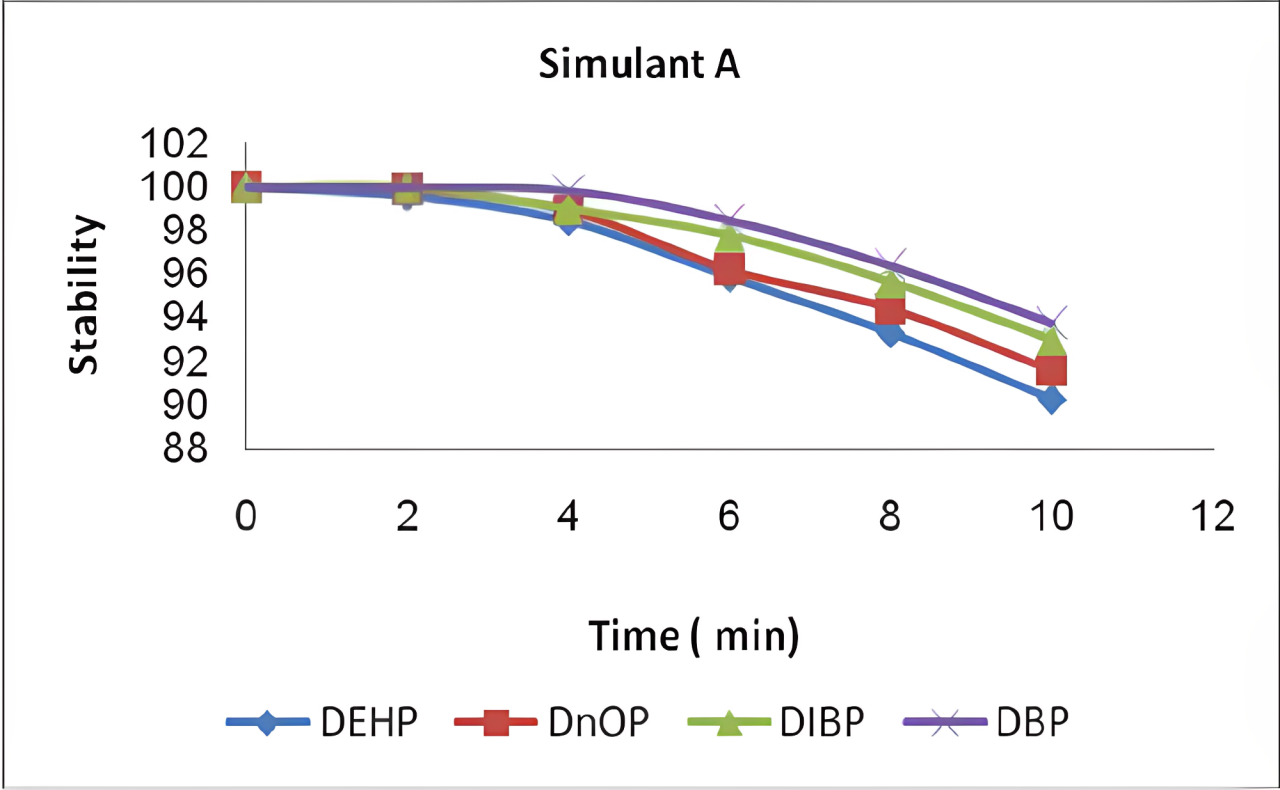
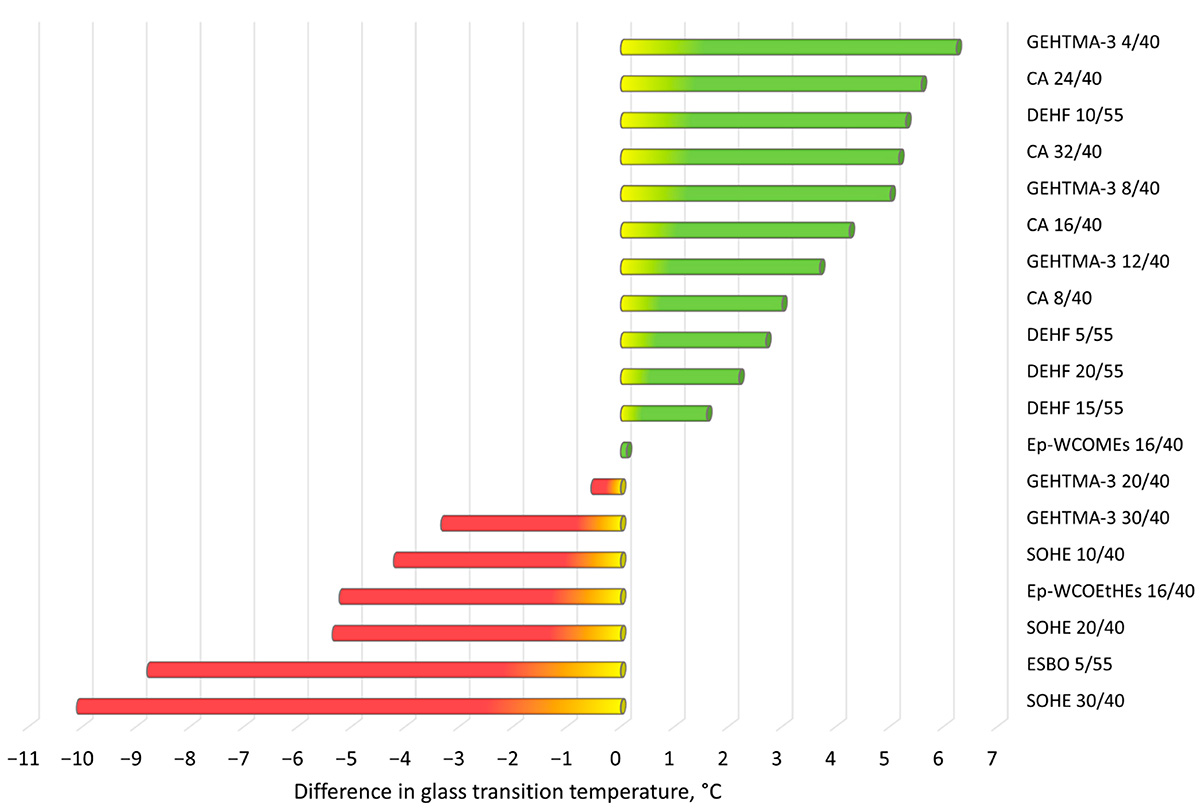
*Adapted from Effect of the microwave heating on the stability of phthalate plasticizers… in aqueous food simulants on ResearchGate, the chart shows phthalate plasticizers’ stability under microwave – induced aqueous food simulant conditions.
(Click to Enlarge)
VI. Durability
Most plasticizers cannot form chemical bonds with polymers. The resin-plasticizer composition remains in a dynamic state, where polymer molecules continuously combine and separate. Therefore, plasticizers tend to migrate out of the plasticized composition. This migration is often more severe, especially in flexible PVC products—or in cases where a large amount of plasticizer is used.
During the service life of plasticized products, plasticizers migrate out of the products primarily through the following three pathways:
- Volatilization: Plasticizers volatilize from the product surface and dissipate into the air.
- Extraction: Plasticizers migrate from the product surface into liquids that come into contact with it.
- Migration: Plasticizers migrate from the product surface into solids that come into contact with it.
Thus, the durability of plasticizers includes volatility resistance, extraction resistance, and migration resistance. The durability of a plasticizer is closely related to its molecular weight and molecular structure. To achieve good durability, the relative molecular weight of the plasticizer generally needs to be greater than 350.
1. Volatility
The volatility of a plasticizer refers to its tendency to escape from the interior of the plasticized material into the surrounding air. The volatilization process first involves migration from the interior of the product to its surface, followed by evaporation from the surface into the air.
Volatility is closely linked to the plasticizer’s molecular weight and molecular structure:
- Generally, plasticizers with lower molecular weights exhibit higher volatility.
- Meanwhile, plasticizers with better compatibility tend to be more volatile.
- Plasticizers with bulky groups in their molecules have low volatility, as these groups hinder their diffusion within the plasticized material.
Among commonly used phthalates, dioctyl phthalate (DOP) has relatively high volatility. Therefore, when using this plasticizer, consideration should be given to the impact of its volatility on production operations.
2. Extraction Resistance
Extraction resistance refers to the tendency of plasticizers to migrate from the interior of a plasticized polymer product into a liquid medium when the product comes into contact with that liquid. Plasticizers can be extracted by a variety of liquids; thus, when plasticized products are in contact with liquids, measures should be taken to retain the plasticizers within the products.
The degree of extractability depends on two key factors:
On one hand, it is determined by properties of the plasticizer, such as its structure, polarity, and molecular weight; on the other hand, it is influenced by the nature of the liquid medium in contact with the plasticized material.
Notably, the water resistance and soap resistance of plasticizers are inversely related to their oil resistance: plasticizers with a higher proportion of alkyl groups in their molecules exhibit better water resistance and soap resistance.
Polyester plasticizers are known for excellent durability. Their properties vary slightly depending on the raw materials used and the type of end groups, but molecular weight has the most significant impact on their performance. High-molecular-weight polyester plasticizers offer good volatility resistance, extraction resistance, and migration resistance, but they have poor cold resistance and plasticizing efficiency.
3. Migration Resistance
Migration of plasticizers refers to the process by which plasticizers diffuse into solid media. During this process, plasticizers diffuse from the plasticized material (where their concentration is high) through contact points into another polymer (such as another plastic or rubber) that it comes into contact with.
The migration of plasticizers is relative to the solid medium they contact. Migration often causes issues such as softening, stickiness, or even surface cracking of the plasticized material, while also leading to product contamination.
The migration tendency of plasticizers is closely related to both the plasticizer’s own structure and the polymer medium it contacts:
- For phthalate plasticizers, migration decreases sharply as the length of the aliphatic chain increases.
Plasticizers exhibit high migration to nitrocellulose and ABS, but low migration to polypropylene (PP), polyethylene (PE), and polystyrene (PS).
VII. Insulation Properties
Pure PVC resin exhibits excellent electrical performance, and rigid PVC products have extremely high-volume resistivity. However, this resistivity gradually decreases with the addition of plasticizers.
The insulation properties of PVC plasticized materials are reflected by parameters such as resistivity, dielectric constant, power factor, and voltage breakdown strength—among which volume resistance is the most commonly used indicator. Generally, volume resistance varies significantly with the type of plasticizer, and insulation performance gradually decreases as the amount of plasticizer increases.
There is an inverse relationship between cold resistance and electrical performance: plasticizers with good cold resistance typically have poor electrical performance. This is because plasticizers with lower polarity allow greater freedom of movement for dipoles on the polymer chain, which increases electrical conductivity and reduces electrical insulation. On the other hand, plasticizers with more branched chains in their molecules (and thus poor plasticizing efficiency) tend to have better electrical performance. For example, dioctyl adipate (DOA) causes the most significant reduction in the volume resistance of plasticized materials, while chlorinated paraffins are typical representatives of insulating plasticizers.
The purity of plasticizers is closely related to the electrical performance of plasticized materials. Charged ionic substances easily migrate within plasticized materials; therefore, if a plasticizer contains ionic impurities, the electrical insulation performance will decrease significantly.
Ⅷ. Flame Retardancy
PVC is a polymer with a chlorine content of 56%, which itself has flame retardancy and self-extinguishing properties. If it is used in combination with plasticizers with good flame retardant performance, its flame retardancy will be even better. However, if it is used in combination with ordinary flammable plasticizers, the PVC plasticized material will no longer be flame-retardant. Flame retardancy can usually be expressed by afterflame time or oxygen index. A longer afterflame time means better flame retardancy, and a higher oxygen index means better flame retardancy. Generally speaking, the flame retardancy of plasticizers is affected by the following aspects:
(1)The volatility of the plasticizer in the polymer. The greater the volatility, the worse the flame retardancy.
(2)The decomposition products generated during combustion. Ideally, the decomposition products should not be combustion-supporting substances; if they are, the flame retardancy will be reduced.
(3)The chemical structure of the plasticizer. When the plasticizer contains phosphorus, chlorine and aryl structures, its flame retardancy is relatively good.
At present, the widely used flame-retardant plasticizers include phosphate esters, chlorinated paraffins and chlorinated fatty acids. The most prominent feature of phosphate ester plasticizers is strong flame retardancy. They are widely used as plasticizers for PVC, and can also produce good flame-retardant effect when used alone as flame retardants. Chlorinated paraffins are low in price and are widely used as secondary plasticizers. The performance of chlorinated paraffins is closely related to their chlorine content. With the increase of chlorine content, both flame retardancy and compatibility are improved, but the cold resistance deteriorates significantly. Chlorinated fatty acids have better compatibility with PVC than chlorinated paraffins.
Ⅸ. Viscosity Stability
Viscosity stability is specific to plasticizer pastes.
Ⅹ. Toxicity
Most plasticizers have some degree of toxicity, varying in severity. DOP is safe when packaged normally, but it may be extracted by oils when in contact with high-fat foods, which should be avoided. DOA has good hygienic properties. Phosphate esters are classified as plasticizers with relatively high toxicity.
Conclusion
That wraps up our overview of plasticizer performance evaluation. In practical work, however, “perfect plasticizers” are rare—for instance, low-molecular-weight plasticizers with high plasticizing efficiency often have high volatility; phosphate esters with good flame retardancy require trade-offs between toxicity and cold resistance. I often tell the younger members of our team that choosing a plasticizer is like “building with blocks”: first, clarify the core needs of the product (do you need it to be soft, tough, or durable?), then screen plasticizers based on key properties, and even use compound formulations if necessary to make up for the shortcomings of a single plasticizer.
Through this article, I’ve tried to translate our team’s years of practical experience in PVC plasticizer selection and formula optimization into accessible technical insights—these aren’t just theoretical concepts, but solutions we’ve verified in real projects, from food-contact PVC packaging to low-temperature-resistant outdoor hoses. If you want to dive deeper into topics like plasticizer compounding techniques or latest industry trends, feel free to visit our blog page, where we share more hands-on case studies and technical breakdowns. And if you’re facing specific challenges with your PVC project—whether it’s fine-tuning plasticizer formulas to meet performance standards or solving issues like migration or low-temperature brittleness—don’t hesitate to send us an inquiry via email: sales@kingstarmold.com. Our team will tailor a solution based on your project’s unique needs, helping you turn technical hurdles into product advantages.
Learn more about plasticizers in our related posts about plasticizers:
Plasticizer: What Is It & How Does It Work?
PVC Plasticizers: Overview & Selection Tips for PVC Applications