The quality of injection molded products is the cornerstone for enterprises to establish themselves in the market, and quality managers are the core guardians of this quality defense line. From pre-production preparation to shift handover, rigorous and standardized operations directly determine the final quality of the product. To help quality managers clearly grasp the key points of work and efficiently carry out quality control, the complete operation guide is now organized as follows, which must be strictly implemented.
- 1. Overview of Inspection Process
- 2. Pre-Production Preparation: Building a Solid Quality Foundation
- 3. First Article Confirmation: Control the Starting Point of Production
- 4. First Article Operation Quality Guidance: Unified Standards
- 5. Inspection Record: Dynamic Monitoring of the Production Process
- 6. Shift Handover: Ensuring Work Continuity
- 7. Basic Management: Asset and Document Control
1. Overview of Inspection Process
Pre-production preparation → First Article Confirmation → Inspection and Defective Product Inspection → Pre-delivery Inspection → Defective Product Confirmation → Shift Handover
2. Pre-Production Preparation: Building a Solid Quality Foundation
According to the Injection Molding Daily and Weekly Production Plan, the following preparations should be made in advance to ensure an orderly start to the inspection work:
- Prepare all relevant mastersamples: injection molded parts, color chips, color samples, appearance boundary sample, dimensional mastersamples;
- Collect a complete set of technical documents: Injection Molded Parts Drawing, Injection Molded Parts Inspection Standards, Injection Material Details, Process Card, Mold Modification Notice, Design Change Order;
- Prepare testing fixtures such as vernier calipers, depth gauges, inner diameter calipers, etc., and confirm that their accuracy meets the standard;
- Prepare product accessories for subsequent assembly inspection.
3. First Article Confirmation: Control the Starting Point of Production
3.1 Timing of First Article Confirmation
The first article confirmation must be carried out in the following situations: machine shutdown, mold replacement, material replacement, core replacement, process adjustment, and shift handover.
3.2 Basis for First Article Confirmation
The core judgment criteria are based on the Injection Molded Parts Drawing, Parts Inspection Standards, Injection Material Details, and the development of master samples, color palettes, and color standards.
3.3 First Article Confirmation Content
① When the process is stable, confirm the appearance, (including color), function, structure, important dimensions, and matching parts of each cavity product in a whole mold, and record the inspection results in the Injection Molding First Article Confirmation Form;
② Parts related to compatibility must be checked for compatibility with relevant mating components;
③ When the size inspection results do not match the standard, but match the master sample, the master sample shall be used for judgment;
④ After the first article confirmation is OK, notify the Production Department to start mass production and sign the QC sample of this batch of parts for reference by the operators (placed in a fixed position on the machine).
3.4 Abnormal Feedback on First Article Confirmation
① If any quality or material abnormalities are found during the first article confirmation process, they should be immediately isolated, labeled, and the team leader and production manager should be notified for improvement;
② If the on-site team leader’s machine adjustment exceeds 50 cycles and the problem is still not solved, the production manager should be notified immediately. If the problem is not solved within 1 hour and affects the product function, production should be stopped;
③ If quality and process abnormalities cannot be immediately resolved after being handled by the team leader or factory manager, QC should issue a Quality Exception Report Form if necessary. After being signed by the general manager, it should be handed over to the Production Department or Quality Control Department for handling. If the situation is serious and needs to be stopped, the injection molding team leader or factory manager should be notified, and the team leader should be required to sign the Injection Molding First Article Confirmation Form;
④ After receiving the anomaly report, the responsible department should analyze the cause of the anomaly and propose improvement measures, and reply to the proposing unit and the relevant units for implementing the measures;
⑤ QC should establish an exception record and track the handling status, and confirm the closure of the case based on the results.
4. First Article Operation Quality Guidance: Unified Standards
Targeted coaching will be provided based on categories such as electroplated parts, welded parts, mating parts, and colors. The core content is as follows:
4.1 Operation Techniques
Clearly define the key operation procedures for product observation, handling, trimming range and degree, wiping position and method, etc., to ensure that operators have a clear understanding.
4.2 Quality Requirements for Classified Products
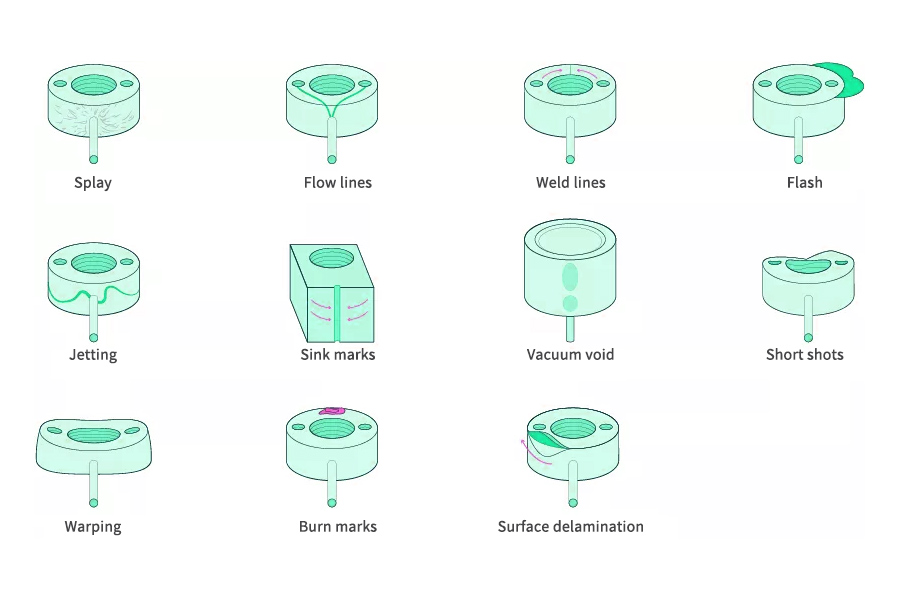
- Appearance Parts – Electroplated Products:Common defects include pitting, cold slug, splay, flow marks, sink marks, flow lines, weld lines, etc. Severely defective products can be selected, polished, or scrapped, while slightly defective products are acceptable and require on-site sampling for reference and judgment by operators; Electroplated parts with poor appearance need to be polished and marked.
- Compatibility Requirements: Products that need to be matched shall undergo compatibility testing every hour, and if necessary, 100% actual matching shall be carried out. The actual matching standard shall refer to the first article.
- Internal Components – Sealing Surface:There should be no scratches, mold strains, sink marks, obvious weld lines or other concave and convex defects, and the dimensions should be within the tolerance range.
- Internal Components – Welding Surface:There should be no obvious sink marks, scratches, strains, splay, ejector pin marks, or raised ejector pins, and all welding ribs should be full and without gaps.
- Internal Component Compatibility: Products that require compatibility shall undergo compatibility checks every hour, and 100% inspection shall be conducted if necessary.
4.3 Special Precautions
① When producing various types of fast food containers, it is necessary to check whether the products are prone to cracking, whether the wall thickness around them is uniform, whether the assembly with matching parts has a good sealing effect, and what problems have occurred or may occur in previous production.
② Products with high quality requirements are classified as Level 1 products, while products with normal requirements are classified as Level 2 products. The overall dimensions should be checked for consistency.

4.4 Packaging and Protection Specifications
- Packaging Requirements:Refer to the List of Packaging Bases and guide operators to master the correct packaging bases and methods.
- Product Protection:Handle with care to avoid collision, scratches, and damage. When bagging, ensure that it is properly placed and not exposed. When packing, do not exceed the edge of the box.
4.5 Verification of Coaching Effectiveness
Specific coaching projects can also refer to the Quality Coaching for First Article Operators for the various requirements and operating methods that you have explained. After the explanation, it is necessary to verify whether the operator understands what you have said. You can check whether the products that have been inspected by the operator are qualified. If there are any unclear areas, you must re explain and demonstrate until the operator understands and can do them.
5. Inspection Record: Dynamic Monitoring of the Production Process

5.1 Basic Requirements for Inspection
QC shall inspect the machine at least once every two hours, with no less than four mold products randomly selected each time. The following tasks shall be emphasized:
- Product Inspection: Based on the drawings, mastersamples, and part inspection standards, inspect the appearance (including color), dimensions, structure, function, and compatibility, and record the results in the Injection Inspection Record Form.
- Operation Specification Inspection: Check whether the employee’s operation method is correct, whether mutual inspection is done well, and whether standardized operation is followed. If non-standard operations are found, they will be handled according to the degree of quality impact: notify the operator and supervisor for the first time; Notify the operator, supervisor, and injection molding supervisor for the second time; Notify the operator, quality control supervisor, and production plant manager for the third time.
- Process and Document Inspection: Confirm that the machine parameters are consistent with theProcess Card, and notify the supervisor to adjust if they exceed the tolerance range; If the adjustment according to the process card cannot meet the requirements and the process needs to be changed, the team leader should be notified and the factory manager should be informed, and the process card should be updated synchronously. At the same time, check whether the machine is complete and placed with documents such as Process Card, Machine Inspection Form, Inspection Standards, Operation Manual, etc.
5.2 Sampling and Judgment Criteria
- In the operator’s self inspection of qualified products, if the number of functional and structural non-conforming products is ≥ 2PCS, or the number of appearance non-conforming products is ≥ 3PCS, it is judged that the products from the previous period are non-conforming.
- In the selection or post-processing of products by operators, if the number of non-conforming products or scrap products is ≥ 3PCS, it is judged that the products from the previous period are non-conforming.
- After the inspection is judged as unqualified, QC needs to record it in the Injection Inspection Record Form, and after confirmation and signature by the supervisor, arrange for the operator to isolate and rework; After rework, it must pass the operator’s self inspection before being sent for inspection.
- For the full box products selected by the operator, after passing the sampling inspection, stamp the QC seal and confirm whether the information such as part number, name, quantity, date, and special identification is correct; If the sampling inspection fails, a rework seal shall be affixed and the team leader and operator shall be notified to rework.
5.3 Abnormal Feedback and Handling
(1) Abnormalities that can be immediately resolved: Record in detail the abnormal points, improvement measures, and handling results in the Injection Inspection Record Form.
(2) Abnormalities that cannot be immediately resolved or cross time periods: issue a Quality Exception Report, submit the Appearance Exception Report to the General Manager for judgment, and submit the Functional and Structural Exception Report to the Production Department for processing after confirmation by Quality Control; Abnormal orders are distributed by quality control to relevant departments for implementation, and QC is responsible for tracking the response and implementation of countermeasures.
5.4 Confirmation of Defective Products
- Before each inspection and shift handover by QC, it is necessary to randomly check the scrapped boxes to confirm whether there are any good products mixed in with the defective ones, and promptly handle any problems found.
- Defective products with less than 20 molds can be scrapped after confirmation by the injection molding factory and QC; For molds exceeding 20 times, they must be confirmed by the injection molding workshop, quality inspection, and production department, and approved by the general manager before being scrapped. When necessary, the quality control department shall issue a Quality Exception Reportto the injection molding workshop, which shall be confirmed by the production department and submitted to the injection molding workshop for analysis of the cause, formulation of countermeasures, and QC tracking and verification.
6. Shift Handover: Ensuring Work Continuity
- The incoming QC shall hold the Handover Record, communicate and confirm with the QC of the previous shift one by one, inspect the quality status, first articleand other key information of each machine in the previous shift one by one, and hand over any abnormal situations clearly. If necessary, feedback shall be given to the production department according to regulations.
- Any abnormalities that occur in this shift, their handling results, and work precautions should be recorded in detail in the Handover Recordand fully handed over to the next shift.
- All types of forms, documents, and other important matters received on the same day must be registered and handed over in the Handover Record.
7. Basic Management: Asset and Document Control
(1) Properly archive documents and records; Strengthen the control of samples, testing fixtures, and QC seals, regularly maintain and store them, and ensure that all resources are traceable and in good condition.
(2) Quality exception handling closed-loop process
- OK Process:Quality Control Department issues notification → Injection Molding Workshop distributes → Responsible Department executes improvement measures → Quality Control Department tracks execution results → General Manager confirms effectiveness.
- NG Process:Quality Control Department issues notification → Injection Molding Workshop hands over to Production Department for processing → Quality Control Department distributes → Responsible Department executes improvement measures → Quality Control Department tracks execution results → General Manager confirms
Quality control is no trivial matter, and every rigorous and meticulous step is a powerful guarantee for product quality. We hope that all quality managers will strictly follow this guide to carry out their work, proactively identify and solve problems in a timely manner, and jointly build the quality defense line of the enterprise. If the content of the guide needs to be adjusted based on actual production, feedback and optimization can be provided at any time.
For quality managers, the most severe challenges often stem from persistent problems that repeatedly occur and are difficult to eradicate through on-site adjustments. As a leading injection molding company in China, KingStar is well versed in this field, and we focus on starting from the root of mold design and process to provide you with radical solutions. If you need to overcome quality bottlenecks and enhance product competitiveness, please feel free to contact us by email (sales@kingstarmold.com) or fill out a from on our contact page.



